Recently, demand for energy efficiency, reduced carbon emissions, and improved performance has driven development of lighter automotive and aerospace components.
 Chris GoodeLight metals like magnesium are becoming crucial to achieve new standards in component weight reduction; however, magnesium and its alloys need protective surface treatments to achieve acceptable performance and service life. Plasma Electrolytic Oxidation (PEO) is an enhanced form of anodization that applies greater current potential than standard anodizing to develop chemical, thermal, and plasma reactions on the substrate to form a thick, dense ceramic oxide coating. Manufacturers frequently apply PEO coatings to improve the mechanical properties of light metals and passivate them against corrosion; however, current PEO techniques typically employ toxic chemicals, and the plasma process is energy intensive.
Chris GoodeLight metals like magnesium are becoming crucial to achieve new standards in component weight reduction; however, magnesium and its alloys need protective surface treatments to achieve acceptable performance and service life. Plasma Electrolytic Oxidation (PEO) is an enhanced form of anodization that applies greater current potential than standard anodizing to develop chemical, thermal, and plasma reactions on the substrate to form a thick, dense ceramic oxide coating. Manufacturers frequently apply PEO coatings to improve the mechanical properties of light metals and passivate them against corrosion; however, current PEO techniques typically employ toxic chemicals, and the plasma process is energy intensive.
This article introduces an innovative, low voltage, sustainable PEO technology. Designated Cirrus Guardian, this novel PEO process offers a sustainable coating for magnesium components at reduced cost, with much lower energy requirements, and a high degree of process control. Developed specifically for magnesium (but adaptable to other light metals), the Guardian PEO process deposits a uniform, tightly bonded protective layer from a benign bath at low current density and achieves high deposition rates.
Conventional Plasma Electrolytic Oxidation (PEO) Processes
Plasma electrolytic oxidation (PEO) is a surface modification process that generates ceramic layers on light metal alloy surfaces. PEO-generated coatings are distinguished by their corrosion resistance, wear resistance, thermal and chemical stability, and are two to four times harder than anodized surfaces. To create a PEO coating, a high voltage, often 300 – 500 V, is applied to the substrate against a counter electrode, causing plasma discharges on the surface (main image). The plasma vaporizes the metallic substrate and redeposits the metal as a (mostly) crystalline oxide. Conventional PEO coating processes are energy intensive and frequently rely on harmful chemicals such as chrome or fluorine-based compounds.
Responding to industry demand for sustainable surface finishing technology, Cirrus has developed the novel Guardian PEO process to offer an efficient protective coating for magnesium components with 50% - 70% lower energy needs and that is deposited from a non-toxic electrolyte. The Guardian PEO process generates thick, adherent, and corrosion-resistant coatings on Mg alloys at voltages as low as 85 V, while the novel incorporation of nitrides in the coating enables a further 10% - 15% increase in surface hardness compared to conventional PEO coatings.
Synthesis/ Sustainable Bath Preparation
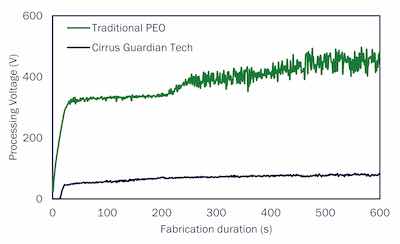 Figure 2: Time voltage curve during Guardian PEO coating of AZ80 Mg alloy. The voltage remains below 90 V for the entire coating process.The Guardian process for magnesium uses an alkaline bath comprised of environmentally friendly chemicals such as sodium silicate, sodium citrate, hydrogen peroxide, a surfactant, and a non-toxic amino phenolic compound. This novel bath chemistry readily forms low porosity corrosion-resistant oxides and silicates into the PEO-generated coatings. The patented Guardian process also includes addition of aminophenols to the electrolyte to synthesize carbide and nitride content in the coatings, further lowering porosity and improving mechanical performance.
Figure 2: Time voltage curve during Guardian PEO coating of AZ80 Mg alloy. The voltage remains below 90 V for the entire coating process.The Guardian process for magnesium uses an alkaline bath comprised of environmentally friendly chemicals such as sodium silicate, sodium citrate, hydrogen peroxide, a surfactant, and a non-toxic amino phenolic compound. This novel bath chemistry readily forms low porosity corrosion-resistant oxides and silicates into the PEO-generated coatings. The patented Guardian process also includes addition of aminophenols to the electrolyte to synthesize carbide and nitride content in the coatings, further lowering porosity and improving mechanical performance.
Most existing literature on PEO surface treatments report using processing voltages in the range of 200 – 500 V 1-4. Figure 2 shows the time vs voltage curve for a Guardian low-voltage PEO coating compared to a more traditional PEO coating, here the voltage always remains below 100 V. Cirrus Guardian technology adopts novel conductive bath chemistry that supports a low-voltage PEO process to develop microcrack-free ceramic coatings on light metals. In addition, these less energy-intensive processes are easier to control than the ones that rely on >200 V for PEO treatment, allowing multiple substrates to be coated simultaneously. An added benefit of using low voltages is the eco-friendliness of Guardian technology.
Surface Morphology / Structure
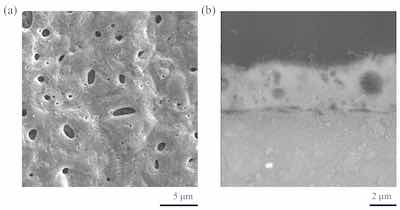 Figure 3: Surface (a) and cross-section (b) images of Guardian PEO coatings on AZ80 Mg alloys showing reduced porosity and no observable micro-cracking.Unlike conventional PEO surfaces, which can be characterized by a random pore distribution and micro-cracking, Guardian PEO surfaces exhibit a dense, basalt-like morphology with few pores and no observable micro-cracks, as illustrated in the SEM images at Figure 3. This result is directly due to the addition of aminophenol surfactants to the PEO electrolyte which both reduce the size of the arc discharge bubbles and increase the temperature and pressure at the surface, depositing a denser, less porous, and crack free coatings.
Figure 3: Surface (a) and cross-section (b) images of Guardian PEO coatings on AZ80 Mg alloys showing reduced porosity and no observable micro-cracking.Unlike conventional PEO surfaces, which can be characterized by a random pore distribution and micro-cracking, Guardian PEO surfaces exhibit a dense, basalt-like morphology with few pores and no observable micro-cracks, as illustrated in the SEM images at Figure 3. This result is directly due to the addition of aminophenol surfactants to the PEO electrolyte which both reduce the size of the arc discharge bubbles and increase the temperature and pressure at the surface, depositing a denser, less porous, and crack free coatings.
Mechanical Behavior
PEO coatings typically offer 1 – 3x the surface hardness of untreated light metals5. However, because of the increased density of the Guardian PEO surface, Cirrus measured surface hardness values above 7 GPa, more than 7X that of a standard surface. The formation of carbides, nitrides, and oxynitrides in the Guardian PEO composition further enhances the mechanical properties of the surface.
Corrosion Behavior
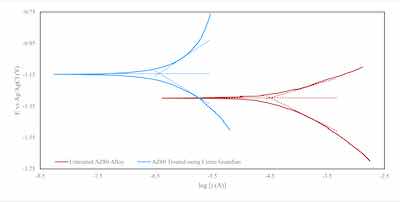 Figure 4: Tafel curves obtained from potentiodynamic testing of an untreated and Cirrus Guardian treated AZ80 alloys.Despite their weight and structural properties, light alloys are vulnerable to corrosion. As typical PEO coatings exhibit high porosity and micro-cracking, they often require post-processing to achieve satisfactory corrosion resistance. Guardian PEO coating differs as the development of nitrides during the coating formation adjusts the sacrificial corrosion potential of the coating relative to that of a bare magnesium substrate.
Figure 4: Tafel curves obtained from potentiodynamic testing of an untreated and Cirrus Guardian treated AZ80 alloys.Despite their weight and structural properties, light alloys are vulnerable to corrosion. As typical PEO coatings exhibit high porosity and micro-cracking, they often require post-processing to achieve satisfactory corrosion resistance. Guardian PEO coating differs as the development of nitrides during the coating formation adjusts the sacrificial corrosion potential of the coating relative to that of a bare magnesium substrate.
Figure 4 shows the Tafel curves for an untreated AZ80 magnesium alloy and one coated using Cirrus PEO technology. Table 1 provides the extracted corrosion potential value as –1140 mV for PEO-treated alloys compared to --1298 mV obtained for an untreated AZ80 alloy. The corrosion current for PEO-treated alloys is nearly 100 times lower than that of untreated alloys. Our hypothesis is that the densified nitride coatings on Mg alloys, produced by the Guardian process, improves their corrosion resistance.
Table 1: Summary of corrosion potentials extracted from the Tafel plots in Figure 4 for untreated and Cirrus PEO tech treated AZ80 alloys.
| Ecorr (mV) | icorr (μA/cm2) | |
| Untreated AZ80 Alloy | -1290 | 32.2 |
| AZ80 Alloy Treated using Cirrus PEO Tech | -1140 | 0.38 |
Composition
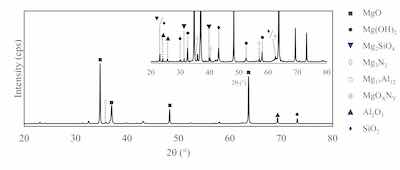 Figure 5: XRD analysis of Guardian PEO on AZ80 (inset - enlarged low-intensity peaks).Cirrus Guardian PEO technology generates plasma energies sufficiently high to form of Mg3N2 and magnesium oxynitride despite the reduced energy consumption. Figure 5 shows the X-ray diffraction (XRD) patterns obtained from PEO-treated AZ80, while the inset shows the graphical magnification of the low-intensity peaks that are not easily visible. XRD analysis shows the coating is a composite of magnesium oxides, hydroxides, and silicates. The pattern also shows the presence of alumina and silica, nitrides and oxynitrides of magnesium. This novel composition offers coatings with superior mechanical and corrosion performance from a low energy coating process.
Figure 5: XRD analysis of Guardian PEO on AZ80 (inset - enlarged low-intensity peaks).Cirrus Guardian PEO technology generates plasma energies sufficiently high to form of Mg3N2 and magnesium oxynitride despite the reduced energy consumption. Figure 5 shows the X-ray diffraction (XRD) patterns obtained from PEO-treated AZ80, while the inset shows the graphical magnification of the low-intensity peaks that are not easily visible. XRD analysis shows the coating is a composite of magnesium oxides, hydroxides, and silicates. The pattern also shows the presence of alumina and silica, nitrides and oxynitrides of magnesium. This novel composition offers coatings with superior mechanical and corrosion performance from a low energy coating process.
Photocatalytic Performance
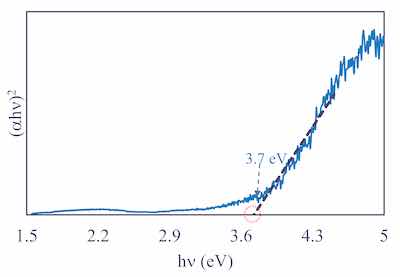 Figure 6: Tauc plot generated from the reflectance spectrum collected for Mg alloy treated using Cirrus Guardian technology. The estimated bandgap of 3.7 eV is remarkably lower than the 7.5 eV observed for commercially available MgO.Mg-based ceramic materials are typically wide-bandgap semiconductors with indirect bandgaps of 7.5 to 9 eV. The novel composition achievable with Guardian PEO processes exhibits a low bandgap of 3.7 eV. Figure 6 shows the Tauc plot extracted from reflectance data collected for a Mg alloy treated using Guardian PEO processes, suggesting that bulk nitride and silicate containing Mg oxide-based Guardian coatings can potentially function as antimicrobial surfaces.
Figure 6: Tauc plot generated from the reflectance spectrum collected for Mg alloy treated using Cirrus Guardian technology. The estimated bandgap of 3.7 eV is remarkably lower than the 7.5 eV observed for commercially available MgO.Mg-based ceramic materials are typically wide-bandgap semiconductors with indirect bandgaps of 7.5 to 9 eV. The novel composition achievable with Guardian PEO processes exhibits a low bandgap of 3.7 eV. Figure 6 shows the Tauc plot extracted from reflectance data collected for a Mg alloy treated using Guardian PEO processes, suggesting that bulk nitride and silicate containing Mg oxide-based Guardian coatings can potentially function as antimicrobial surfaces.
Conclusion
Many well-known PEO processes still rely on toxic chemicals and high energy consumption to protect light metal alloys such as magnesium. The patented Guardian PEO technology designed by Cirrus Materials Science overcomes the sustainability drawbacks of conventional PEO coating processes, offering high quality coating outcomes and significantly lower energy use from a non-toxic and low-cost bath chemistry. Guardian PEO coatings are thick, demonstrate excellent adhesion, and provided corrosion resistance for magnesium components.
The Guardian PEO process can be adapted to a variety of applications and the functionality of the coating can be readily adjusted with additives. The bath chemistry can support multiple light metal options, including multi-material components. With these features, Guardian PEO technology offers an environmentally friendly, versatile, and high performing breakthrough surface design.
Methods
1. Synthesis
a. Substrate preparation and pre-treatment of Magnesium AZ80 alloys
Prior to PEO coating the sample surfaces were prepared by mechanically roughening the substrate using emery paper followed by cleaning in a commercially available 80°C alkaline bath, for 15 mins. The pre-treatment process ensured the removal of organic contamination from machined substrates. The alkaline bath comprises 20 g/L NaCO3, 20 g/L Na2PO4, 20 g/L Na2SiO3, and 3 g/L OP-10 surfactant. Finally, we used DI water to rinse the substrate. We designed the cleaning process to limit any buildup of the native oxide layer on the substrate.
b. PEO coating process
Mg alloys were coated in a 25°C PEO bath comprising 45 g/L NaOH, 60 g/L Na2SiO3, 10g/L Na3C6H5O7, 6 mL/L H2O2, 0.05 milli mol/L sodium dodecyl sulfate (SDS) and 5 mL/L aminophenol. Alkaline bath chemistry modified with H2O2 ensured the generation of oxide coatings. A silicate compound was used to incorporate silicate content into the coating and enhance the conductivity of the bath. Citrate compounds aided in the uniform distribution of surface arc generation. SDS was utilized to moderate the physical properties of the PEO bath to improve the removal of gas bubbles generated during processing. The treatment process was conducted for 15 minutes using a stainless-steel counter electrode. A variable voltage DC (Direct Current) power system supplied a constant current of 1 A/dm2 resulting in an average processing voltage of <150 V.
2. Nano-indentation
The mechanical behavior of the ceramic oxide coatings was analyzed using a Hysitron TI 950 tribometer equipped with a Berkovich tip. PEO coating cross-sections were mounted in epoxy for the nanoindentation tests to eliminate any interference from the underlying substrate. A maximum load of 1000 mN was applied for 2 seconds with 5-second preloading and unloading times. Nanoindentation tests on non-oxidized control substrates aided in evaluating the mechanical enhancement provided by the ceramic coating.
3. Scanning Electron Microscope
FEI XL30 SEM equipped with a 30 kV field emission gun was used to analyze the surface morphologies and composition of the PEO- coatings. Prior to imaging, the samples were sputtered with Pt using a Quorum Tech Q150T turbomolecular pumped coater to improve their electron conductivity for SEM imaging.
4. XRD
A Rigaku XtaLAB Synergy-s single crystal X-ray diffractometer equipped with a Cu Ka source (l = 1.542 Å, 2q= 20° to 80°, 0.02° step size) was used to collect phase and composition data on the coated sample surfaces. The XRD patterns were examined using the Materials Explorer application on the Materials Project open database. [39]
5. Corrosion Behavior
To evaluate the electrochemical performance of the PEO coatings a CH Instruments three-cell electrochemical workstation equipped with a Metek designed K0235 flat cell kit was used. The samples were immersed in 3.5 wt.% NaCl solution for 30 minutes, prior to testing. The tests employed an Ag/AgCl reference electrode and a Pt counter electrode. A fresh-prepared 3.5 wt.% NaCl solution was used for Tafel and EIS tests. The open circuit potential (OCP) of the coatings were scanned from –0.3 V to 0.3 V for 5 minutes for Tafel tests.
6. Photocatalytic Performance
Diffuse reflectance spectra for PEO coatings on Mg were collected using a Shimadzu 2250 UV-Vis spectrophotometer. The photonic energies ranged from 1.5 to 6.2 eV (l = 200 to 800 nm) with a medium scan speed and 0.016 eV (0.5 nm) resolution. The flat samples were irradiated with a 5° photonic incidence angle via the specular reflectance attachment.
Chris Goode is Chairman and CTO at Cirrus Materials Science in Auckland, New Zealand, a nano-technology and surface finishing specialist that designs new coating technologies for harsh environments and high-performance applications. Visit https://cirrusmaterials.com
References
- Dong, K. H., Song, Y. W., Shan, D. Y. & Han, E. H. Formation mechanism of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Surface & Coatings Technology 266, 188-196, doi:10.1016/j.surfcoat.2015.02.041 (2015).
- Shanaghi, A., Mehrjou, B. & Chu, P. K. Enhanced corrosion resistance and reduced cytotoxicity of the AZ91 Mg alloy by plasma nitriding and a hierarchical structure composed of ciprofloxacin-loaded polymeric multilayers and calcium phosphate coating. J. Biomed. Mater. Res. Part A, 16, doi:10.1002/jbm.a.37258.
- Nie, X., Wang, L., Konca, E. & Alpas, A. T. Tribological behaviour of oxide/graphite composite coatings deposited using electrolytic plasma process. Surface & Coatings Technology 188, 207-213, doi:10.1016/j.surfcoat.2004.08.025 (2004).
- Cai, J. S. et al. The preparation and corrosion behaviors of MAO coating on AZ91D with rare earth conversion precursor film. Applied Surface Science 257, 3804-3811, doi:10.1016/j.apsusc.2010.11.153 (2011).
- Tu, X. H., Miao, C. P., Zhang, Y., Xu, Y. L. & Li, J. Y. Plasma Electrolytic Oxidation of Magnesium Alloy AZ31B in Electrolyte Containing Al2O3 Sol as Additives. Materials 11, 11, doi:10.3390/ma11091618 (2018).



































