Ogden Air Logistics Center (OO-ALC) is the primary facility within the United States Air Force for maintaining and overhauling aircraft landing gear.
Aluminum landing gear components are anodized at OO-ALC to provide enhanced corrosion resistance, paint adhesion, and wear resistance; a sodium dichromate sealing operation usually completes the anodizing process. During sealing, the pores of the anodized (oxide) layer are hydrated, which fills the pores and provides improved corrosion resistance. However, this sealer contains hexavalent chromium, which is listed on the Environmental Protection Agency’s list of industrial toxic chemicals that are targeted for voluntary reduction or elimination.
The specification that outlines the sodium dichromate sealing process delineates three alternative processes that are approved for use: 1) boiling de-ionized water, 2) cobalt acetate, and 3) nickel acetate. While some research to support the use of these and other sealing processes has been gathered under past efforts, additional work must be conducted to fully integrate non-chromate sealers into OO-ALC’s anodizing operations.
To meet this need, the Air Force Research Laboratory tasked Concurrent Technologies Corporation to identify viable alternatives to the sodium dichromate sealer, conduct testing on these alternatives, and recommend the most promising sealer(s) for implementation based on the test results. This paper will describe the requirements for anodizing and sealing operations within OO-ALC, as well as the sealing technologies that are available and a path forward to demonstrate/validate the most promising alternatives for the specific needs and applications of OO-ALC.
Introduction
 C-5 Galaxy Aircraft.Anodizing is a mature and commonly used electrochemical process that controls and enhances the formation of protective oxide coatings on aluminum (Al) and Al alloys. The process is used for a wide variety of commercial and military applications. For example, anodizing operations are routinely performed at the Ogden Air Logistics Center (OO-ALC) at Hill Air Force Base, Utah, as part of the depot maintenance process on numerous vehicles and weapon systems within the United States Air Force (USAF) inventory. Specifically, OO-ALC is the primary facility within the USAF for maintaining and overhauling aircraft landing gear. Al landing gear components are anodized at OOALC to provide wear and corrosion-resistant surfaces. A photo of the C-5 Galaxy aircraft, as well as the main landing gear from this aircraft, is presented in Figure 1 [Refs. 1, 2, 3].
C-5 Galaxy Aircraft.Anodizing is a mature and commonly used electrochemical process that controls and enhances the formation of protective oxide coatings on aluminum (Al) and Al alloys. The process is used for a wide variety of commercial and military applications. For example, anodizing operations are routinely performed at the Ogden Air Logistics Center (OO-ALC) at Hill Air Force Base, Utah, as part of the depot maintenance process on numerous vehicles and weapon systems within the United States Air Force (USAF) inventory. Specifically, OO-ALC is the primary facility within the USAF for maintaining and overhauling aircraft landing gear. Al landing gear components are anodized at OOALC to provide wear and corrosion-resistant surfaces. A photo of the C-5 Galaxy aircraft, as well as the main landing gear from this aircraft, is presented in Figure 1 [Refs. 1, 2, 3].
A sealing operation usually completes the anodizing process. During the sealing process, the micropores of the anodized (oxide) layer are hydrated. Because the hydrated form of the oxide has a greater volume than the unhydrated form, this process fills the micropores and subsequently improves corrosion and staining resistance [Ref. 4]. The most commonly used sealing process, a sodium dichromate solution, has been used for many years and has proven to be both effective and robust. A standard dichromate sealer bath can last a year or longer with standard loading. However, this solution contains hexavalent chromium (Cr6+). Note: Cr6+ is a hazardous material that is listed on the Office of the Secretary of Defense (OSD) Emerging Contaminants Action List, the Environmental Protection Agency’s (EPA’s) list of toxic industrial chemicals to be voluntarily reduced or eliminated, and in Superfund Amendments and Reauthorization Act (SARA) Title 313 [Toxic Release Inventory (TRI)]. Additionally, the Department of Defense (DoD) issued a policy directive in April 2009 that restricts the use of Cr6+ compounds on military vehicles and weapon systems [Ref. 5]. Therefore, the DoD has an ongoing need to identify and validate a chromium-free sealer to reduce the use of Cr6+ in anodizing operations, thereby reducing associated environmental and compliance burdens.
 Main landing gear.MIL-A-8625F is the military specification that establishes the requirements for anodizing and sealing processes used for military components [Ref. 6]. This specification delineates three alternative sealing processes that are approved for use: boiling deionized (DI) water, solutions containing cobalt acetate, and solutions containing nickel acetate. However, sufficient data to promote the use of these and other chromium-free sealing processes do not exist.
Main landing gear.MIL-A-8625F is the military specification that establishes the requirements for anodizing and sealing processes used for military components [Ref. 6]. This specification delineates three alternative sealing processes that are approved for use: boiling deionized (DI) water, solutions containing cobalt acetate, and solutions containing nickel acetate. However, sufficient data to promote the use of these and other chromium-free sealing processes do not exist.
To address these issues, the Air Force Research Laboratory (AFRL) Energy and Environment Team (RXSCP) tasked Concurrent Technologies Corporation (CTC) to identify and demonstrate/validate an alternative to sodium dichromate sealers used in anodizing operations at OO-ALC. The successful implementation of a chromium-free sealing technology will reduce environmental compliance burdens and foster enhanced worker safety at OOALC. Under this effort, numerous interviews with OO-ALC personnel were conducted. The purpose of the interviews was to identify the technical requirements for the parts being processed and to capture the desired characteristics of an alternative sealer. CTC then identified relevant products by conducting Internet searches, vendor interviews, and literature reviews.
This article presents the findings from the interviews with ALC personnel (requirements analysis) and product search (technology assessment) conducted during this effort. Although this activity focused on the specific needs of OO-ALC (e.g., military components and applications), the information and methodology presented herein are useful for anyone interested in exploring alternatives to dichromate sealers for anodizing operations.
Anodizing and Sealing Requirements
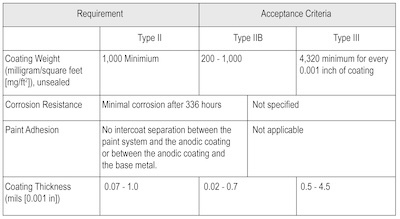 Table 1. MIL-A-8625F Performance Requirements for Anodic Coatings on Aluminum Alloy Components.OO-ALC uses sulfuric acid (Type II and Type IIB) and hard coat (Type III) anodizing at its facility. Therefore, processing and coating requirements for Types II, IIB, and III anodizing at OO-ALC were determined. Based on interviews with OO-ALC personnel, anodizing processes at OO-ALC are used to treat components made from 2000, 6000, and 7000 series Al alloys. The performance requirements for the three types of anodizing conducted at OO-ALC must meet the requirements of MIL-A-8625F, which are summarized in Table 1 [Ref. 6].
Table 1. MIL-A-8625F Performance Requirements for Anodic Coatings on Aluminum Alloy Components.OO-ALC uses sulfuric acid (Type II and Type IIB) and hard coat (Type III) anodizing at its facility. Therefore, processing and coating requirements for Types II, IIB, and III anodizing at OO-ALC were determined. Based on interviews with OO-ALC personnel, anodizing processes at OO-ALC are used to treat components made from 2000, 6000, and 7000 series Al alloys. The performance requirements for the three types of anodizing conducted at OO-ALC must meet the requirements of MIL-A-8625F, which are summarized in Table 1 [Ref. 6].
The acceptance criteria recognize that corrosion resistance is determined using the standard salt spray test in accordance with ASTM B117 [Ref. 7], and that paint adhesion is determined in accordance with Federal Test Method Standard 141, Method 6301 [Ref. 8]. (1See Table 2: Acceptance Criteria for Corrosion Resistance.) .
Sulfuric Acid (Type II and Type IIB) Anodizing
OO-ALC uses sulfuric acid (Type II and Type IIB) anodizing to anodize the components of various aircraft landing gear. Current is applied when parts enter into the solution and are gradually increased to the desired current density (typically 12 to 22 volts). The coatings become harder and denser as the purity of the aluminum in the substrate increases [Ref. 4].
Parts that have been anodized with the Type II and IIB processes are sealed with the sodium dichromate sealer; therefore, these processes were the primary focus of this study. The main Environmental Health and Safety (EHS) concerns expressed by OO-ALC personnel for these processes are the corrosive nature of the sodium dichromate solution and the fact that it contains Cr+6.
Hard Coat (Type III) Anodizing
OO-ALC performs the hard coat (Type III) anodizing process after conducting Type II anodizing on a part as part of their aircraft refurbishment processes. Because Type III anodized coatings are sealed in boiling DI water, the primary EHS concern is thermal exposure to the boiling water. Therefore, this process was not a focus of this study.
Sealing Process and Acceptance Criteria
OO-ALC personnel reported that OO-ALC has two sodium dichromate sealer tanks for sealing anodized parts. Both of these tanks contain solutions with a concentration of 5 to 9 ounces per gallon (oz/gal) sodium dichromate. The pH is maintained between 5.0 and 6.3 with sodium hydroxide, and the temperature is maintained between 95–98 degrees Celsius (°C) (203–208 degrees Fahrenheit [°F]). Considering the requirements of MIL-A-8625F and the needs identified by OO-ALC, acceptance criteria for an alternative sealing process for Type II and Type IIB anodize were defined, as listed in Table 2 [Ref. 6].
Table 2. Acceptance Criteria for OO-ALC Anodized Parts Sealed with Sodium Dichromate
| Test | Test Method | Acceptance Criteria |
| Coating Weight (prior to sealing) | ASTM B137 [Ref. 9] | 1/000 mg/ft2 minimum for Type II 200 mg/ft2 minimum – 1/000 mg/ft2 maximum for Type IIB |
| Corrosion Resistance | ASTM B117 [Ref. 7] | a. No more than a total of 15 isolated pits/ none larger than 0.031 inch in diameter in a total of 150 square inches of test area grouped from five or more test pieces (areas within 0.062 inch from identification markings, edges and electrode contact marks remaining after processing shall be excluded), and; b. No more than 5 isolated pits none larger than 0.031 inch in diameter in a total of 30 square inches from one or more test pieces (areas within 0.062 inch from identification markings, edges and electrode contact marks remaining after processing excluded). |
| Wet Tape Adhesion | FED-STD-141/ Method 6301 [Ref. 8] | No intercoat separation between the paint system and anodic coating or the anodic coating and the base metal. |
| Dry Tape Adhesion | ASTM D3359/ Method B [Ref. 10] | No intercoat separation between the paint system and anodic coating or the anodic coating and the base metal. |
OO-ALC personnel confirmed that the above tests would be sufficient to test alternatives for their acceptance as candidates to replace the sodium dichromate sealer.
Technology Assessment: Available Alternatives to Sodium Dichromate Sealers
To identify candidate technologies that meet the requirements described above (technologies that may be promising for future study), a thorough technology assessment was conducted. The following activities were undertaken:
Review of past efforts that evaluated sealers for anodized coatings, specifically those focused on sodium dichromate replacement.
- Internet searches
- Discussions with vendors
- Literature search(es)
Processes undergoing research and development (R&D), as well as commercially available processes, were considered. For processes that were evaluated under past efforts, vendors were contacted to obtain the most recent information regarding their products. This assessment also identified recently developed or improved inorganic sealers that would require technology verification testing if found to be promising.
As expected, a number of technologies were identified for sealing anodized Al alloys. These alternatives are based on a variety of materials, such as nickel acetate, silicon, cobalt and molybdenum fluorides, and trivalent chromium, among others. Prior work in this field [Ref. 11] has classified the more prominent sealing processes into general categories that include:
- Water-based sealing processes, such as boiling DI water and steam.
- Chromate sealing
- Sodium silicate sealing
- Nickel-based sealing technologies, such as nickel acetate and cold nickel fluoride
The additional category of cobalt-based sealers was identified during the current effort. A special assessment also was performed for emerging technologies. Each of these categories is briefly described in the following sections.
Water-Based Sealing Technologies
Water-based technologies have been a viable method for sealing anodized coatings for many years. The performance of the seal is dependent upon the pH, temperature, and purity of the water, as well as immersion time and current density used during anodizing [Refs. 4, 11]. DI water is commonly used for this reason. Past work has shown that hot water sealing may be the best sealing process for enhancing the dielectric strength of anodized coatings on Al [Ref. 12], which is essential for applications requiring electrical conductivity [Ref. 13].
Water-based sealing technologies work by converting aluminum oxide (anodic coating) to boehmite, which fills the micropores of the anodic coating. The mechanism is presented in Equation 1 [Ref. 11]:
Al2O3 + H2O 2AlO(OH) (1)
Steam sealing is a variation of boiling DI water sealing. This technique is used frequently in Japan and Europe and is generally more efficient than boiling DI water immersion, with a reported reaction rate increase of two to four times that of DI immersion [Ref. 11]. However, steam sealing requires specialized equipment, and it is rarely employed in the United States due to the high capital investment and production costs.
Because boiling DI water is approved as a sealer in MIL-A-8625F and is already used at OO-ALC for Type III anodizing operations, it was a viable sealer for Types II and IIB anodizing. As such, it was considered to be a baseline process for future testing.
Chromate Sealing
Sodium dichromate solutions have been used as seals for anodized coatings for many years. The abilities of chromates to inhibit corrosion are well recognized and have been reported by Brooman [Ref. 14], Klingenberg [Ref. 15], and numerous others. Unlike water-based sealing technologies, chromate sealing technologies form either aluminum oxydichromate (lower pH ranges) or aluminum oxychromate (higher pH ranges) in the coating micropores [Ref. 11].
Cr+6 is used in most chromate sealing systems, especially those used for military applications, due to the enhanced corrosion protection that they impart. The Cr+6-containing sodium dichromate solution (5–9 oz/gal) being used at OO-ALC was identified as the baseline being targeted for replacement. The bath temperature for this seal is maintained at 90°C –100°C (194°F –212°F), and the pH is maintained between 5.0 and 6.0; immersion time is 15 minutes [Refs. 6, 11]. It is noted that because OO-ALC is actively trying to reduce the use of or replace chromium-containing processes, even sealers based on trivalent chromium, which have been developed in recent years and are available and being used commercially, were not considered under this pollution prevention effort. However, it was determined that a sealing solution with a reduced sodium dichromate content (50 parts per million [ppm] as chromium) could be evaluated as a benchmark because successful results had been achieved in past work [Ref. 16] and reducing the Cr+6 content in the existing solution would be an interim contribution to OO-ALC’s goal for chromium reduction.
Sodium Silicate Sealing Technologies
Sodium silicate sealing is a mature, effective sealing technique that is commonly used for Type III anodizing. The bath temperature is maintained at 85°C –95°C (185°F –203°F), and the pH is maintained at around 11.0; immersion time is 10-15 minutes [Ref. 11]. Like hot water sealing, silicate sealing converts aluminum oxide to boehmite (Eq. 1) [Ref. 11]. While promising for some applications, these processes are reported to be inferior with respect to corrosion resistance [Ref. 11] to other types of sealing processes, specifically hot water, dichromate, and nickel-based sealers. In addition, silicate sealers are not recommended for applications that require dielectric strength [Ref. 12]. Furthermore, silicate is recognized as being detrimental to conventional anodizing electrolytes, as contamination of the anodizing bath with silicates reduces the corrosion resistance of the resulting coating [Ref. 17]. Due to these limitations, as well as a lack of available information to justify the consideration of silicate sealing technologies, these types of sealers were not considered for further study.
Nickel-Based Sealing Technologies
A number of nickel-based sealing technologies are available. These are usually based on either nickel acetate (approved for use under MIL-A8625F) or nickel fluoride. Nickel acetate sealing is one of the most predominant sealing processes in U.S. anodizing operations due to the superior corrosion resistance it imparts [Refs. 4, 11]. Nickel fluoride sealing technologies have been adopted for some applications but, reportedly, do not perform as well as nickel acetate sealers [Ref. 11].
The mechanism for nickel acetate sealing appears to be more complex than that of hot water or silicate sealing. Along with the aluminum oxide conversion to boehmite (Equation 1), the precipitation of nickel hydroxide also occurs, as described in Equation 2 [Ref. 11]:
Ni2+ + 2OH Ni(OH)2 (2)
These concurrent precipitation reactions (e.g., Equations 1 and 2) fill the micropores.
While nickel-based sealing processes are technically promising, nickel has also come under increased scrutiny from an EHS standpoint in recent years. Nickel is considered a carcinogen by the International Agency for Research on Cancer (IARC) and the National Toxicology Program (NTP), although it is not yet classifiable as a human carcinogen by the American Conference of Governmental Industrial Hygienists (ACGIH) [Ref. 18]. Nevertheless, the Occupational Safety and Health Administration (OSHA) maintains a long-standing permissible exposure limit (PEL) on the use of the soluble salts of nickel at one milligram per cubic meter (mg/m). Perhaps most importantly, nickel is on the OSD Emerging Contaminants Watch List [Ref. 19].
Despite these concerns, numerous nickel-based sealing technologies were identified under this activity, and at least three of these were found to be promising based on the review of the technical literature. Based on the maturity and availability of nickel-based sealers, as well as their approval under MIL-A-8625F, a commercial-off-the-shelf (COTS) nickel-based sealer product was selected as a candidate. This product operates at a lower temperature than the sodium dichromate sealer (29–35°C [85–95°F]), contains no chromates, and has low nickel content (5-10% by weight). Recommended immersion (sealing) time is between 4 and 15 minutes. It is noted that, as expected, the sealing reaction occurs much slower in lower-temperature seals than in higher-temperature seals. It has been found that a warm water rinse (71°C [160°F]) accelerates the sealing process [Ref. 4]. With this in mind, the COTS nickel-based seal was considered in two scenarios: with warm water rinse afterward and without the rinse.
Cobalt-Based Sealing Technologies
Cobalt-based sealers are also approved as a dichromate sealer replacement under MIL-A-8625F; however, these sealers appear to be a relatively new development, and sufficient data to support using cobalt as a sealer were not available. Even some process data, such as optimum immersion time and temperature, were not readily available. In addition, cobalt is on the OSD Emerging Contaminants Watch List [Ref. 19]. Based on these considerations, cobalt-based sealing technologies were not considered to be viable candidates for OO-ALC sealing requirements at this time. Therefore, they were not considered for further study.
Emerging Technologies
Several additional technologies were found to be promising under this effort, based on a review of past work [Refs. 20–23], as well as vendor surveys.
The Non-Chrome Post Treatment (NCP) process was found to be promising as a non-chromium seal for anodizing. NCP is a proprietary product of the United States Naval Air Systems Command (NAVAIR). Testing was conducted by NAVAIR under past work [Ref. 20], and the results demonstrated exceptional performance in corrosion-resistance testing and adhesion testing. While these results demonstrate that the NCP sealer might be viable for the needs of OO-ALC, the process was unavailable for demonstration at the time of testing; therefore, it could not be considered.
A COTS chromium and nickel-free anodized sealer based on permanganate was identified that reportedly provides characteristics similar to those of the dichromate sealer. This solution was evaluated by the AFRL and CTC under past work [Ref. 20] and showed exceptional performance in corrosion resistance. This sealer also passed the dry tape adhesion test but failed the wet tape adhesion test.
Sealing anodized coatings by using a liquid colloidal dispersion that solidifies (e.g., sol-gel) has been demonstrated. Specimens were anodized and then immersed in a working solution at 270°C for 15 minutes; the resulting specimens reportedly demonstrated comparable corrosion resistance to dichromate-sealed specimens [Ref. 22]. However, this process is more expensive and decreases the abrasion resistance and hardness of the overall coating system. The incorporation of additional materials within the sealer, such as polytetrafluoroethylene (PTFE), has also been explored [Ref. 23]. These technologies are typically used for low friction and/or “nonstick” applications; sufficient data that these sealers could meet the stringent needs of military specifications could not be obtained, and so they were not considered.
Demonstration/Validation Testing
Based on the findings of this activity, three candidates were selected for laboratory testing: the COTS permanganate solution, the COTS low nickel sealing solution, and a 50 ppm sodium dichromate solution that will serve as a benchmark. As mentioned previously, the COTS low nickel seal will be considered with and without a warm water rinse after the sealing process. The currently used sodium dichromate sealer, the boiling DI water seal, and unsealed options will be evaluated as baselines for all testing conducted for this effort. The candidate test matrix is summarized in Table 3.
Table 3. Test Matrix: Sodium Dichromate Sealers and Most Promising Seals to Evaluate for Replacement
| Sealer | Additional Information |
| Sodium dichromate solution 5-9 oz/gal | Baseline |
| Boiling deionized water | Baseline |
| Unsealed | Baseline |
| Sodium dichromate solution 50 parts per million (ppm) | Benchmark |
| COTS permanganate solution | Candidate |
| COTS low nickel seal solution with hot water seal | Candidate |
| COTS low nickel seal solution without hot water seal | Candidate |
Efforts to test aluminum alloy test panels that have been anodized and sealed with the above processes are in progress.
(2 The ideal processing temperature of the NCP process is 100°F, and the optimum processing time is 10 minutes. 3 The solution was made up of 10% by volume. Temperature was maintained at 170°F -180°F, pH was maintained between 5.0 and 8.0, and seal time was 12 to 15 minutes.)
Summary
The overall objective of this effort was to identify alternatives to the sodium dichromate sealer used in anodizing operations. To this end, the requirements for the sodium dichromate sealer currently in use at OO-ALC were determined. Furthermore, extensive literature and vendor searches identified promising candidates that may meet the requirements. The findings were then used to derive a path forward to demonstrate/validate the most promising alternatives for the specific needs and applications of OO-ALC.
Future Work
Having established the test methods and acceptance criteria for replacing sodium dichromate sealers, as well as available candidates for testing, the effort will focus on testing and data evaluation. It is anticipated that this work will identify a candidate that can meet the needs of OO-ALC with respect to an anodizing seal. Once this candidate has been identified, thoroughly validated through testing, and recommended for use at OO-ALC, the process of modification of the relevant technical order (TO) for conducting anodizing and sealing operations at OO-ALC will be initiated accordingly. The replacement of sodium dichromate in this application will reduce the environmental burden and increase worker health and safety at OO-ALC.
This paper was originally published in 2011; the authors might have changed career positions since then.
About The Authors
 Rob Mason is a Senior Materials and Process Scientist at Concurrent Technologies Corporation (CTC) in Largo, Fla. In this role, he provides technical support to CTC’s clients in the government and commercial industry. His current work includes providing support to inorganic coating activities conducted by the Air Force Research Laboratory (AFRL). Mr. Mason has more than 20 years of cumulative experience in surface engineering, coatings R&D, and testing and evaluation methodology development and has co-authored over 50 technical publications and presentations on these subjects. He earned his B.S. in Chemistry from Fairleigh-Dickinson University, New Jersey. He is a member of the National Association of Surface Finishers (NASF), NACE International, and Toastmasters International.
Rob Mason is a Senior Materials and Process Scientist at Concurrent Technologies Corporation (CTC) in Largo, Fla. In this role, he provides technical support to CTC’s clients in the government and commercial industry. His current work includes providing support to inorganic coating activities conducted by the Air Force Research Laboratory (AFRL). Mr. Mason has more than 20 years of cumulative experience in surface engineering, coatings R&D, and testing and evaluation methodology development and has co-authored over 50 technical publications and presentations on these subjects. He earned his B.S. in Chemistry from Fairleigh-Dickinson University, New Jersey. He is a member of the National Association of Surface Finishers (NASF), NACE International, and Toastmasters International.
 Sarah Clark is an Associate Chemical Engineer at CTC in Dayton, Ohio. Her main responsibilities are providing project management and technical project support to various inorganic coating-focused projects. Ms. Clark is the subtask lead for the Dichromate Sealer Replacement Subtask of the Inorganic Finishing Technologies Task Order for the AFRL. She is responsible for providing budget management and technical planning, as well as leadership and oversight to the technical staff supporting the Subtasks. Ms. Clark holds a Bachelor of Science in Chemical Engineering from the University of Dayton with a minor in Environmental Engineering.
Sarah Clark is an Associate Chemical Engineer at CTC in Dayton, Ohio. Her main responsibilities are providing project management and technical project support to various inorganic coating-focused projects. Ms. Clark is the subtask lead for the Dichromate Sealer Replacement Subtask of the Inorganic Finishing Technologies Task Order for the AFRL. She is responsible for providing budget management and technical planning, as well as leadership and oversight to the technical staff supporting the Subtasks. Ms. Clark holds a Bachelor of Science in Chemical Engineering from the University of Dayton with a minor in Environmental Engineering.
 Dr. Melissa Klingenberg is an Advisor Engineer at CTC in Johnstown, Pa. She provides overall support to the Environmental Technologies Discipline with particular emphasis on the laser decorating and inorganic finishing areas. Her primary inorganic finishing responsibilities lie in innovative coating and surface finishing processes, with her areas of expertise being advanced vacuum deposition/surface finishing technologies and plating processes. Dr. Klingenberg received a B.S. in Chemistry and engaged in post-baccalaureate studies in biology at the University of Pittsburgh at Johnstown. She received an M.S. in Manufacturing Systems Engineering at the University of Pittsburgh and a Ph.D. in Materials Engineering at the Pennsylvania State University. Dr. Klingenberg has been an active member of NASF since 1994 and has presented and/or written numerous papers for NASF and AESF events and publications.
Dr. Melissa Klingenberg is an Advisor Engineer at CTC in Johnstown, Pa. She provides overall support to the Environmental Technologies Discipline with particular emphasis on the laser decorating and inorganic finishing areas. Her primary inorganic finishing responsibilities lie in innovative coating and surface finishing processes, with her areas of expertise being advanced vacuum deposition/surface finishing technologies and plating processes. Dr. Klingenberg received a B.S. in Chemistry and engaged in post-baccalaureate studies in biology at the University of Pittsburgh at Johnstown. She received an M.S. in Manufacturing Systems Engineering at the University of Pittsburgh and a Ph.D. in Materials Engineering at the Pennsylvania State University. Dr. Klingenberg has been an active member of NASF since 1994 and has presented and/or written numerous papers for NASF and AESF events and publications.
Dr. Elizabeth Berman is a Senior Research Engineer in the Materials & Manufacturing Directorate of the AFRL at Wright-Patterson Air Force Base, Ohio. She has 17 years of experience with the Air Force, two years with the Navy, and 10 years in private industry. Dr. Berman is currently assigned to the Environment and Energy Quality Team. She is the AFRL lead and manager for programs to reduce cadmium and chromium in metal plating operations. She is also the AFRL lead on aircraft and runway deicing technology programs and the technical manager for both fluid and non-fluid methods to eliminate hazardous pollutants used in aircraft and runway deicing. Additionally, Dr. Berman is leading the Air Force effort on algae research to manage carbon dioxide sequestration concerns. Dr. Berman has two years of experience managing aircraft painting systems and processes at the Naval Aviation Depot in Alameda, Calif. She also worked for three years at the Air Force Center for Environmental Excellence, where she represented the Air Force in technical negotiations with base, contractor, regulator, and community representatives on more than 25 Air Force installations.
Dr. Natasha Voevodin is a Senior Research Engineer with the University of Dayton Research Institute, working for the Environmental and Energy Quality Team in the Materials & Manufacturing Directorate of the AFRL. She has more than 20 years of experience in materials research, specializing in coating formulation, corrosion science, chemical analyses, and spectroscopic techniques. She has a strong background in the material science of organic, inorganic, and polymer coatings application of analytical and spectroscopic techniques. Her most recent work includes programs to reduce hazardous materials in metal plating operations, the development of advanced inorganic finishing technologies, environmental and energy technology evaluations, and the evaluation of environmentally benign coating removal processes.
Acknowledgments: This work was conducted under tasking by the Air Force Research Laboratory. The authors would like to express thanks to Mr. Tom Naguy for his support of this work. Additionally, the authors would like to express thanks to Ms. Ruth Schaefer, Ms. Laurie Swenson, and Mr. Nathan Hughes of OO-ALC for their time and consideration in support of this effort. The authors also appreciate the efforts of Ms. Donna Provance of CTC, not only for her outstanding leadership but also for her technical editing of this document. Finally, the authors would like to recognize Dr. Eric Brooman for his outstanding work in the field of metal finishing during many years of service and wish him well in his well-deserved retirement.
References
1. University of New Hampshire website: http://pubpages.unh.edu/~rrt23 /finalproject5.html
2. S. Clark et al, “Requirements for Replacement of Dichromate Sealer for U.S. Air Force Anodizing Operations,” presentation to SUR/FIN 2010, Grand Rapids, MI, June 2010.
3. Hill Air Force Base website: http://www.hill.af.mil/library/factsheets/factsheet.asp?id=5753
4. C. Grubbs, “Anodizing of Aluminum,” Metal Finishing 2009/2010 Guidebook and Directory, Elsevier Inc., New York, 2009, p. 370.
5. J. Young, “Minimizing the Use of Hexavalent Chromium,” Memorandum for Secretaries of the Military Departments, Office of the Under Secretary of Defense, Washington, D.C., April 8, 2009.
6. MIL-A-8625F, Military Specification, Anodic Coatings for Aluminum and Aluminum Alloys, Department of Defense, 10 September 1993.
7. ASTM B117, Standard Test Method for Operating Salt Spray (Fog) Testing Apparatus, ASTM International, West Conshohocken, PA.
8. Federal Test Method Standard 141, Paint, Varnish, Lacquer and Related Materials: Methods of Inspection, Sampling and Testing, Method 6301, Adhesion (Wet) Tape Test
9. ASTM B137, Standard Test Method for Measurement of Coating Mass per Unit Area on Anodically Coated Aluminum, ASTM International, West Conshohocken, PA.
10. ASTM D3359, Standard Test Methods for Measuring Adhesion by Tape Test, Method B, Cross-Cut Tape Test, ASTM International, West Conshohocken, PA.
11. L. Hao and B. Cheng, “Sealing Processes of Anodic Coatings – Past, Present, and Future,” Metal Finishing, Elsevier Inc., December 2000, pp. 8-18.
12. T. Westre, “Performance Results for Sealed Type III Anodic Coatings,” SUR/FIN 2000, June 2000.
13. Southern Aluminum Finishing website: http://www.saf.com/content.php?action=showPage&pid= 69&cat_id=12
14. E. Brooman, “Corrosion Protection of Environmentally Acceptable Alternatives to Cadmium and Chromium Coatings: Cadmium, Part I & Part II,” Metal Finishing, Elsevier Inc., Part I April 2000, Part II June 2000.
15. M. Klingenberg et al., “Update on Developments in Cr-free Coatings for Aerospace Applications,” SUR/FIN 2008, June 2008.
16. BAC 5884, Anodizing of Aluminum Alloys, Boeing Process Specification, April 12, 1995.
17. J. Hogue and J. French, Evaluation of the Effects of Contamination by Silicates of Anodizing Seal Water, Final report for Contract Number DAAJ0173C0378, July 1973.
18. R. Straw, “USAF Nickel Plating Issues,” presentation to SERDP Pollution Prevention Technology Thrust Area Working Group (March 1999).
19. C. Long, “Update on OSD’s Emerging Contaminants Program,” Air Force Restoration and Technology Transfer Workshop, March 2011.
20. Final Report, Subtask 35G-01: Alternatives to Sodium Dichromate Sealers, Phase II, General Service Administration Contract GS-23F-0061L, 2004.
21. Final Report, Non-Chromate Conversion Coatings and Sealers for Aluminum Alloys, Strategic Environmental Research and Development Program, Project number PP-673, 2000.
22. M. Zemanova and M. Chovancova, “New Approaches for Sealing Anodic Coatings,” Metal Finishing, Elsevier Inc., New York, October 2005, pp. 33-34.
23. A. Kuhn, “PTFE Coating vs. Impregnation,” Metal Finishing, Elsevier, Inc., New York, October 2005, pp. 35-38.





































