With the increased need for "zero rejects" and improved quality by manufacturers and consumers, there has been a corresponding greater demand for the specification of salt spray tests.
 Frank Altmayer MSFThis means of testing is subject to more than 20 operational variables,1 the most critical of which will be addressed here. A brief description of other corrosion-simulating tests is also presented.
Frank Altmayer MSFThis means of testing is subject to more than 20 operational variables,1 the most critical of which will be addressed here. A brief description of other corrosion-simulating tests is also presented.
Some form of the salt spray test has been in existence since 1914. The modern mode of testing was conceived in 1939 by AES and ASTM Committee B3.2 It was hoped that a test employing 20 percent neutral salt spray could be used to predict the corrosion resistance of finished parts in service. However, the procedure received much criticism for its lack of reproducibility and failure to predict field performance.3
AES enlisted the help of General Motors to investigate methods of improving the test. The outcome was a reduction of the salt concentration to 5% by wt, which provided corrosion results in the same period of time (and sometimes less) and was not subject to operational problems such as frequent clogging of nozzles with salt deposits. The 5 percent neutral salt fog test was adopted by ASTM in 1954 as B117-54T and is currently designated B117-73, as re-approved in 1979.
Two other salt spray specifications are Federal Test Method 151 a, Method 811.1, and Military Standard 810, Method 509. Unfortunately, the three specifications do not always agree. For example, a concentration of 5% or 20% salt may be specified, and limits on salt purity range from 0.2 to 0.3 percent total impurities. Moreover, readings for salt pH and specific gravity are recommended at different temperatures. Finally, allowances for salt solution collection rates vary from 0.5 to 3 mL/hr per 80 cm2 of collector area. These variations lead to differing test results and the specifications are therefore not interchangeable.
Main image courtesy of www.aerotechlabs.co.uk
Principle Behind Tests
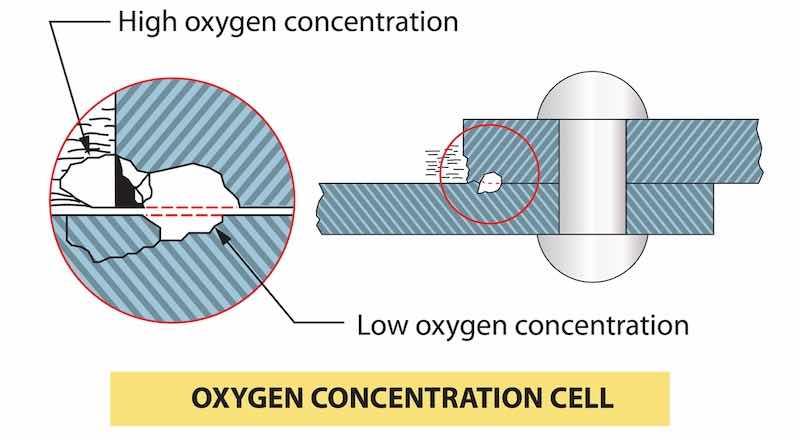 Fig. 1: Oxygen concentration cell corrosion.The salt spray test takes advantage of a phenomenon known as "Oxygen Concentration Cell Corrosion."4 If a drop of water rests on a metal surface (Fig. 1) there is a difference in the volume of oxygen available to the specimen relative to the position within the drop. At the center of the drop, the metal is in contact with the dissolved oxygen in the droplet and with the oxygen in the hydroxide ions due to water dissociation. At the edge of the drop, additional oxygen from the air is available; thus, an oxygen concentration gradient is produced from the edge of the drop to the center.
Fig. 1: Oxygen concentration cell corrosion.The salt spray test takes advantage of a phenomenon known as "Oxygen Concentration Cell Corrosion."4 If a drop of water rests on a metal surface (Fig. 1) there is a difference in the volume of oxygen available to the specimen relative to the position within the drop. At the center of the drop, the metal is in contact with the dissolved oxygen in the droplet and with the oxygen in the hydroxide ions due to water dissociation. At the edge of the drop, additional oxygen from the air is available; thus, an oxygen concentration gradient is produced from the edge of the drop to the center.
The difference in oxygen contact is greatest at the center of the drop. This difference creates an oxidation potential that can be measured and is approximately 0.3 volts.5 The center of the drop/ metal surface becomes anodic (positively charged) as metal dissolves from the water, leaving behind electrons that flow to the edge of the drop. As metal ions are formed at the center of the drop, a "pit" develops.
At the drop's edge, the electrons are available to react with hydrogen ions from the dissociation of water, forming hydrogen molecules (gas). The removal of hydrogen ions results in increased alkalinity at the edge of the drop. The higher alkalinity precipitates the metal ions within the drop, creating the familiar corrosion "ring."
Specimen Preparation
A critical procedure that is often neglected is preparing the parts for exposure. The test specifications indicate that the specimen "shall be suitably cleaned" but do not define this term. The following guidelines are recommended:
- Anodized Aluminum and Decorative Chromium: Wipe with cloth soaked in acetone or alcohol to remove traces of grease and fingerprints from handling.
- Phosphated/Oiled Specimens: Handle as little as possible; touch only at areas that are not significant surfaces. No cleaning should be performed prior to testing.
- Hard Chromium Plate: Clean using magnesium oxide paste until the surface is free of waterbreaks.
- Other Plated Specimens: No cleaning should be employed unless agreed upon by all parties involved.
- Painted Specimens: Protect bare edges by applying paraffin, primer paint or plastic tape. If a performance evaluation upon damage to the coating is desired, score the paint with a sharp cutting edge down to the basis metal.
Exposure of Specimen
 Fig 2: An example of poultice corrosion.Of major concern is the practice of specimen rotation. Rotating the samples within the cabinet every 24 hr is a good idea to ensure that all are exposed to a uniform level of salt fog. Collection rates do vary inside the cabinet.
Fig 2: An example of poultice corrosion.Of major concern is the practice of specimen rotation. Rotating the samples within the cabinet every 24 hr is a good idea to ensure that all are exposed to a uniform level of salt fog. Collection rates do vary inside the cabinet.
However, it is not good practice to rotate about their axis cylindrical parts or those with complicated shapes during exposure. By performing this rotation, the formation of corro- sion cells on the settling surface is interrupted and may even come to a halt (Fig. 2). On the underside of the test specimen, a surface corroded by one primary mechanism (oxygen concentration cell) may now be corroded by a secondary mechanism (poultice corrosion)4 for which the test cabinet was neither designed nor intended.
The exposed specimens can then yield unrealistically high resistance to salt spray because a new surface is exposed each day. (While in service, the parts probably would not be "rotated.") The test results are also difficult to verify in other cabinets because two corrosion mechanisms are used during the exposure.
Practices that contribute to reproducible salt spray results include the following:
- Expose the major surface to be evaluated at a 15° angle from the vertical. The allowable range is 15° to 30°.
- Expose test panels with drilled holes so that the holes are at the bottom.
- Expose test panels parallel to the fog flow pattern. This allows free fog flow between panels.
- Support the test specimen from the bottom whenever possible. Use plastic hooks or coated string only when necessary.
- Do not allow test specimens to touch each other.
- Do not permit spray to impinge directly on the test specimen.
- Do not permit dripping from one specimen onto another.
- Do not interrupt the exposure period except for routine inspection (once a day).
QC and Exposure
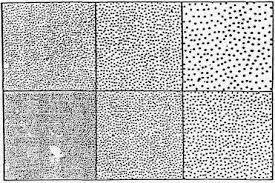 Fig. 3: Ten percent of surface area corroded per ASTM B537. Each square illustrates 10 percent of surface area covered by dots, which vary in size.A minimum quality control program for salt spray testing should include temperature monitoring (and logging) of the cabinet and saturation tower at least once a day (twice daily preferred).
Fig. 3: Ten percent of surface area corroded per ASTM B537. Each square illustrates 10 percent of surface area covered by dots, which vary in size.A minimum quality control program for salt spray testing should include temperature monitoring (and logging) of the cabinet and saturation tower at least once a day (twice daily preferred).
In addition, daily monitoring and logging of collected salt pH, specific gravity and collection rate are important. Weekly titrations for sodium chloride concentration of the collected fog should be performed to verify specific gravity readings.
After exposure in the test chamber, the specimen should be removed with as little handling as possible, rinsed in deionized water at a temperature not greater than 100°F, and dried with compressed air. Samples should not be scrubbed in any manner unless agreed upon by everyone involved.
The exposed specimen can be evaluated using the procedures prescribed in ASTM D610 (painted parts) and ASTM B537 (plated parts). Both employ dot charts (Fig. 3) to indicate the percent area corroded. These charts can be photocopied on plastic transparencies for easy visual comparison.
Frequently, the appearance of the "first sign" of corrosion is cause for failure. For zinc- and cadmium-plated parts and for anodized aluminum, this is specified as "first sign of white corrosion." For steel-based samples, it is the first sign of red rust. It is important to recognize that the first sign of "white" corrosion on cadmium plate can actually appear black due to the presence of cadmium oxide in the corrosion product. The first sign of corrosion on chromated aluminum may also be black. For decorative chromium, it is usually red on steel parts or white on zinc die castings.
Painted panels should be carefully inspected for pinhead-sized blisters that frequently appear before any corrosion is visible. Scored painted panels should be monitored for loss of adhesion from the score line (usually measured in inches).
What Can It Do For Me?
The intent of the salt spray test is to compare the relative corrosion resistance of several specimens or to evaluate the differences between a test sample and a part that has been previously tested and shown to provide satisfactory service. These comparisons should be made with similar coatings. Comparing painted and plated samples, for example, would be invalid.
The test cannot be used to predict service life on a part that has had no previous salt spray/service correlation. Large manufacturers and the U.S. armed forces have developed a correlation between salt spray exposure and service life. Based on these correlations, successful salt spray exposure for a specified period is required before finished products are accepted. Typical exposure times are shown in Table 1.
Table 1: Typical Exposure Periods For Salt Spray Test
| Coating | Exposure; hr |
| Hard chromium | 24-100 |
| Decorative chromium | 24-336* |
| Chromated zinc/cadmium plate | |
| --White corrosion | 24-96 |
| --Red rust | 24-336+ |
| Paint over steel | 500-2000 |
| Galvanized steel | 500-4000 |
| Phosphate/oil | 6 to 24 |
| Anodized aluminum | 96-1000 |
Additional Tests
Other commonly used corrosion tests are outlined below:
- Humidity (ASTM D2247): This test evaluates the corrosion resistance of coated metal products designed for indoor residential use. The corrosion mechanism is the same as that for salt fog testing, except that the absence of salt slows down the reaction.
- Acetic Acid /Salt Spray (ASTM B287): This test is applicable to. painted and plated coatings over steel substrates. It will typically yield faster results than neutral spray due to acid attack (pH 3.1 -3.3).
- CASS (ASTM B368): The Copper Accelerated Salt Spray (CASS) test was the result of AES Research Project 15. It was developed to rapidly test copper/ru'ckel/chromium or nickel/ chromium on steel and zinc die castings for exterior automotive service. It was found that this test more closely duplicated the blisters developed by plated zinc die castings on auto trim. General Motors also discovered that the test correlated well with the service life of painted specimens. The CASS test should not be used on other metallic coatings unless a correlation can be shown. The corrosion mechanism is the same as that for the salt spray test except that the presence of copper, as cupric chloride, adds galvanic potential and the lower pH (3.1-3.3) provides some acid attack.
- Corrodkote (ASTM B380): This test is also a product of AES Project 15 and is intended to simulate the poultice corrosion effects commonly observed on automobiles where adherent, moist dirt is allowed to contact a coated metal part for extended periods of time. It is primarily applicable to parts plated with Cu/Ni/Cr or Ni/Cr. The mechanism is termed poultice corrosion because the paste acts as a "sponge," forming numerous corrosion cells held in place much like dirt on an automobile. The addition of copper, iron, chloride, and ammonium ions creates additional galvanic and chemical effects.
- Sulfur Dioxide (ASTM B605): This test, and also Kester-. nich DIN 50018 (Volkswagen), is used for comparison testing of extremely stable finishes such as tin/nickel or for rapidly detecting a coating defect. The corrosion mechanism is acid attack. Results are usually obtained in 24 hr.
Table 2 compares these tests in brief.
Table 2: Comparison of Commonly Used Corrosion Tests
| Test | ASTM | Coating types | Typical duration | Mechanism | Simulated service condition |
| Humidity Salt | D224 | Various | 24-1000 hr | Oxygen cell | Indoors: mild exposures |
| Salt Spray | B117 | Various | 8-2000 hr | Oxygen cell | Outdoors: moderate to severe exposures |
| CASS | B368 | Cu/Ni/Cr Ni/Cr | 6-720 hr | Oxygen cell plus galvanic | Auto: moderate to severe exposures; parts noj subject to dirt accumulation |
| Corrodkote | B380 | Cu/Ni/Cr Ni/Cr | Three 24-hr cycles | Poultice; chemical attack; galvanic | Auto: severe exposures; parts subject to dirt accumulation |
| Sulfur dioxide | B605 | Sn/Ni & other very stable coatings | 24 hr | Acidic attack | Industrial: stable coatings in severely corrosive environments |
Summary
Salt spray testing and other methods of simulating corrosion performance have grown increasingly important as the call for quality has intensified. An understanding of the mechanism and purpose of each test is essential for obtaining reproducible, accurate results and for correlating these to service conditions.
References
- Frank Alien, Plating, 47, 623 (June 1960).
- "Tentative Method of Salt Spray Testing of Non-Ferrous Metals" (B11 7-39T), ASTM Standards, Supplement, Part 1 (1939).
- H. Sample, "Use and Misuse of the Salt-Spray Test as Applied to Electrodeposited Metallic Finishes," ASTM Bulletin 123, p. 19 and 22 (Aug. 1943).
- Lloyd Gilbert, Corrosion Control course, U.S. Army Wea-pons Command, Rock Island, IL (1975).
- J. Read, Principles of Corrosion, AES Illustrated Lecture (1971).
- H. Uhlig, The Corrosion Handbook, John Wiley & Sons (1948).
- Federal Test Method, Standard 151 a, Method 811.1.
- M. Darsey and W.R. Cavanaugh, "Apparatus and Factors in Salt Fog Testing," Proc. ASTM, 48, 153 (1948).
Frank Altmayer is a Master Surface Finisher and an AESF Fellow, the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in metal finishing. He received the AESF Past Presidents Award, NAMF Award of Special Recognition, AESF Leadership Award, AESF Fellowship Award, Chicago Branch AESF Geldzahler Service Award, and NASF Award of Special Recognition.

































