This study aims to quantify the effect of process parameters on the anodizing of Al6061 aluminum.
To achieve this, studies on layer thickness, the porosity of the anodized surface, electrochemical techniques, X-ray diffraction, grain size estimation, and statistical analysis were conducted for three different atmospheres (without air, air, and oxygen). Parameter levels were established as follows: temperature (30 °C, 45 °C, and 60 °C), time (20 min, 40 min, and 60 min), electrolyte concentration (0.5 M), voltage (9 V), and current intensity (0.600 A). A 33 experimental design (three factors, three levels) was proposed, and mathematical models were obtained using general factorial design. The experimental design was used to determine the three most important variables in the optimal condition.
A total of 27 tests were conducted using sulfuric acid electrolytic solutions, of which 12 samples were selected by the factorial design method, which simultaneously evaluates the effects of factors and their interactions in a single experiment. Measurement of porosity and oxide layer thickness was performed using scanning electron microscopy. The purity of the anodic layer formed was characterized using X-ray diffraction techniques with a vertical goniometer X-ray diffractometer. The electrochemical behavior is presented through potentiodynamic polarization curves for the anodic layer.
A general factorial design and an analysis of variance (ANOVA) were conducted to establish the significant factors for layer thickness, grain size, and reaction rate. Finally, the best results and their parameters for each response are presented.
1. Introduction
Aluminum is one of the most important non-ferrous metals in engineering, used in many chemical and mechanical processes, and produced in both pure form and as alloys. It has a strong affinity for oxygen with the standard molar heat of the formation of Al2O3 of −1,675,700 J at 25 °C (2Al(s)+32O2(g)→Al2O3(s)) [1]. From a physicochemical perspective, the anodizing process explains that when current passes through the electrolyte containing an aqueous solution of sulfuric acid, oxygen is released on the surface of the aluminum connected to the anode. This oxygen allows for the accelerated oxidation of the aluminum. At the cathode (AISI 304 steel), hydrogen is released. In other words, it is a process of water decomposition that generates gases at both electrodes [2]. Ion exchange is the primary mechanism for oxide formation at the metal–oxide interface. When aluminum is polarized as an anode, the basic oxidation reaction that occurs at the interface with the electrolyte can be summarized as follows:
2Al + 3H2O → Al2O3 + 6H+ + 6e− (1)
For anodizing in a sulfuric acid electrolyte, the reaction proceeds as follows:
4Al + 6(H2SO4) → 2Al2O3 + 6(SO−3) + 3(H2)g + 6H+ + 6e− (2)
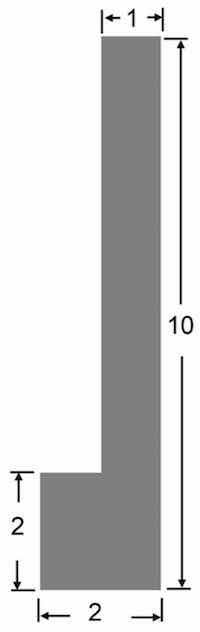 Figure 1. 6061 Aluminum plate geometry (cm).The simultaneous oxidation of aluminum and the electrolysis of the aqueous sulfuric acid electrolyte allows the production of sulfite ions and hydrogen gas at the interface.
Figure 1. 6061 Aluminum plate geometry (cm).The simultaneous oxidation of aluminum and the electrolysis of the aqueous sulfuric acid electrolyte allows the production of sulfite ions and hydrogen gas at the interface.
Due to this high activity, in the presence of oxygen, it reacts in the environment to spontaneously form a thin natural surface layer of alumina (Al2O3), which controls the corrosion rate and protects the metal, enabling the creation of durable aluminum and aluminum alloys [3]. However, it is necessary to increase the thickness of this thin layer to protect the aluminum surface from contact with corrosive agents, because the metallic surface acquires an electric potential that causes the migration of metallic ions into the solution as cations, making the pure aluminum or its alloys prone to corrosion in the electrolyte [4]. The amount of Al2O3 formed during anodizing is directly proportional to the current density and time, while the film’s growth depends on the chemical composition, electrolyte concentration, and anodizing conditions. Some electrolytes have little or no solvent action on the oxide coating, forming very thin films usually known as barrier-type coatings, whose thickness depends only on the applied voltage. This type of coating is typically produced in borate and boric acid solutions. Sulfuric acid is an electrolyte that slightly dissolves the formed coating, achieving the formation of porous films as the oxidation process continues [5]. Sulfuric acid anodizing (H2SO4) was patented by Gower et al. in 1927 for surface finishing and corrosion protection of aluminum and its alloys [6]. Since then, sulfuric acid has become one of the most popular electrolytes for forming a porous oxide on aluminum films, from basic laboratory research to industrial applications, due to the low cost of anodizing electrolytes [6,7,8,9]. It is the most widely used in industry; some researchers have proposed it, obtaining high data on thickness, hardness, and reaction rate [10,11,12].
The corrosion phenomenon occurs because these materials tend to reach their natural state over time, which constitutes a lower potential energy state, allowing them to stabilize thermodynamically with their environment [13]. Most metallic materials tend to show surface changes when exposed to the environment, with the most common changes being color, texture, thickness, chemical composition, and physical properties. Due to this, efforts have been made to counteract the corrosion phenomenon using paints or some electrochemical methods that can delay or prevent its appearance, such as the anodizing process, which is used to improve the surface properties of aluminum by creating a hard oxide layer that is highly wear- and corrosion-resistant [11]. Generally, the microstructure and oxide layer of aluminum depend directly on the effect of the anodizing process variables such as electrolyte nature, concentration, pH, temperature, time, current density, and voltage [14].
Based on the above, this study focused on elucidating a mathematical model for the anodizing process of commercial 6061 aluminum by systematically varying the parameters of time and temperature, with oxygen injection, air injection, and without air injection to improve the mechanical properties and stability of the anodic layer formed on the aluminum, correlating the response through electrochemical techniques such as potentiodynamic polarization.
2. Materials and Methods
2.1. Materials and Pretreatment
In this study, plates of commercial 6061 aluminum 3 mm thick were used; the chemical composition is shown in Table 1. Chemical analysis was performed by optical emission spark spectroscopy Thermo Fisher Scientific, ARL iSpark Plus. Each sample was prepared as shown in Figure 1 for handling within the electrochemical cell of the same material and then sectioned into 2 × 1 cm2 samples for microstructural analysis and corrosion tests.
Table 1. Elemental composition of 6061 aluminum alloy.
| Elemento | S | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Ti | Co | Al |
| % by weight | 0.420 | 0.275 | 0.033 | 0.018 | 0.493 | 0.013 | 0.007 | 0.027 | 0.018 | 0.005 | Bal |
Samples were subjected to mechanical preparation, cleaning in acetone for 5 min, rinsing with distilled water, then drying at room temperature and sanding with silicon carbide paper of grit sizes 320, 500, 800, 1200, and 1500 from lower to higher. Subsequently, they were polished for 3 min on cotton cloth with 1-micron white diamond paste (5 gr) to a mirror finish and stored in a plastic box. To remove impurities in the chemical preparation, they were immersed in a 10% v/v sodium hydroxide (NaOH) solution for one minute, using HNO3 to neutralize the caustic soda effect, and after neutralization, samples were rinsed with distilled water, then dried at room temperature and hermetically stored.
2.2. Anodizing
The anodizing process was carried out under direct energy supply using a two-electrode reactor with an AISI 304 stainless steel plate as the cathode and 6061 commercial aluminum sheets as the anode, maintaining a 2 cm distance between each electrode. During the entire experiment, 50 mL of 5% v/v H2SO4 was used as the electrolyte in each test. The selection of operating variables during the anodizing process is shown in Table 2; fixed and manipulable according to the 32 factorial design (three factors, two levels), and the mathematical model was obtained using the general factorial design.
Table 2. Classification of operating variables 33.
| Operating Variables | Fixed | Manipulable |
| Voltage (V) | ✓ | |
| Current Density (i) | ✓ | |
| Cell Type | ✓ | |
| pH | ✓ | |
| Interelectrode Distance (d) | ✓ | |
| Electrolyte | ✓ | |
| Electrolyte Concentration [ ] | ✓ | |
| Treatment Time (t) | ✓ 20; 40; and 60 min | |
| Electrolyte Temperature (T) | ✓ 30°; 45°; and 60 °C | |
| Agitation Speed (v) | ✓ | |
| Gas | ✓ Without air | |
| Gas | ✓With air | |
| Gas | ✓ With oxygen |
The anodizing process was divided into three steps, using 5% pure sulfuric acid, 9 V voltage, and a current intensity of 0.600 A for each test. In the first step, times of 20, 40, and 60 min were used at temperatures of 30, 45, and 60 °C without air injection. In the second step, the same conditions of time and temperature were applied with air injection using an electric air compressor, HP 1.80, Max Pressure 135 psi, 120 VAC Voltage, and a specific flow rate of 5 lt/min was maintained through control valves. In the third step, oxygen was injected with the same specific flow rate of 5 lt/min and the same conditions of time and temperature. At the end of each step, the anodized electrodes were removed, the anode was washed with 1 mL of ethanol, and then stored in an airtight container, repeating the processes as necessary.
A selection of 12 samples was carried out using the general factorial design method for 3 factors, 2 levels, and high and low levels, as shown in Table 3. This method simultaneously evaluates the effects of the factors and their interactions in a single experiment, allowing for a comprehensive analysis of experimental results and identifying the best level for those factors that influence the mean of the response variable [15]. Table 4 shows the variables manipulated during the anodizing process.
Table 3. General factorial design, 3 factors, 2 levels (32).
| Table | A Low | B Low | C Low |
| 1 | A Low | B Low | C Low |
| 2 | A Low | B High | C High |
| 3 | A High | B Low | C High |
Table 4. Values of the variables manipulated during the anodizing process 33.
| Variable | Low (−1) | Intermediate | High (+1) |
| Temperature (T) | 30 °C | 45 °C | 60 °C |
| Treatment time (t) | 30 min | 40 min | 60 min |
| Gas | No air | No air | No air |
| Gas | No air | With air | With air |
| Gas | With oxygen | With oxygen | With oxygen |
2.3. Structure and Composition of Anodic Films
In this experiment, an X-ray diffractometer with a vertical goniometer (PANalytical, model EMPYREAM) was used to analyze the structure of the anodic films using the grazing incidence technique. The 2θ range was between 20° and 90°, with a scanning speed of 1°/min. The crystallite size was estimated using the Scherrer formula τ = 0.9λ/Bcos (B_max) [16]. The chemical composition of the anodic films was obtained through the integrated analytical platform with Tescan Essence, which combines SEM images with elemental composition analysis using the Tescan Mira 3 LMU scanning electron microscope (SEM). It was performed by energy dispersive spectroscopy (EDS).
2.4. Morphology
The thickness measurement of the anodic layer was performed using a Tescan Mira 3 LMU SEM with a backscattered electron detector (BSE) [17]. The current intensity was standardized at 15 kV with a magnification of 3500×.
2.5. Electrochemical Techniques
For the electrochemical characterization of the anodized aluminum surface, potentiodynamic polarization curves were carried out to determine the current density and corrosion potential. The samples were cleaned with an approximate area of 1 cm2 in a CJ-008 high-frequency ultrasonic cleaner, characterized in a three-electrode cell using anodized aluminum as the working electrode, platinum as the counter electrode, and a saturated KCl Ag/AgCl reference electrode. The tests were performed with a Gamry Instruments Interface 1010E potentiostat, using a 3.5% NaCl solution as electrolyte for one hour, with pH measurements taken before and after each test using a basic Ohaus ST2200-F pH meter.
The open circuit time was 600 s, with a sweep speed of 1.66 mV/s. Cyclic polarization was performed from a 1 V cathode arm to a −1 V anode overpotential with a sweep of 1.66 mV/s.
The adjustment of the Tafel curves obtained makes it possible to determine the cathodic and anodic slopes (βa and βc), and thus determine the corrosion current density (Icorr) and the corrosion rate, calculating the localized corrosion rate (r) in accordance with ASTM G46.
3. Results and Discussion
Micrographs of the SEM show the morphology of the anodic aluminum oxide (AAO) films of aluminum 6061 at times of 30 and 60 min and temperatures of 30 and 60 °C are shown in Figure 2 without air injection. It was used to ensure there was no formation of sulfides and other agents that react with oxygen. It is not for quantifying the film thickness, but to identify that there is not a significant amount of SO2−4 on the surface, contaminant phases in the aluminum oxide film, and to search for phases that may be on the surface of the material, because the formation is thin, but impurities may be present. The same conditions with air injection are shown in Figure 3, and with oxygen injection in Figure 4, following the nomenclature T, t, condition. As shown in Figure 2a, the AAO surface formed at 30-20-SA has an irregular and non-uniform surface. Figure 2b–d show uniform and stable layers formed at 60-20-SA, 30-60-SA, and 60-60-SA, respectively. The thickness of the layer increases with shorter times, with an average thickness of 5.47 μm in the cross-section of samples without air injection.
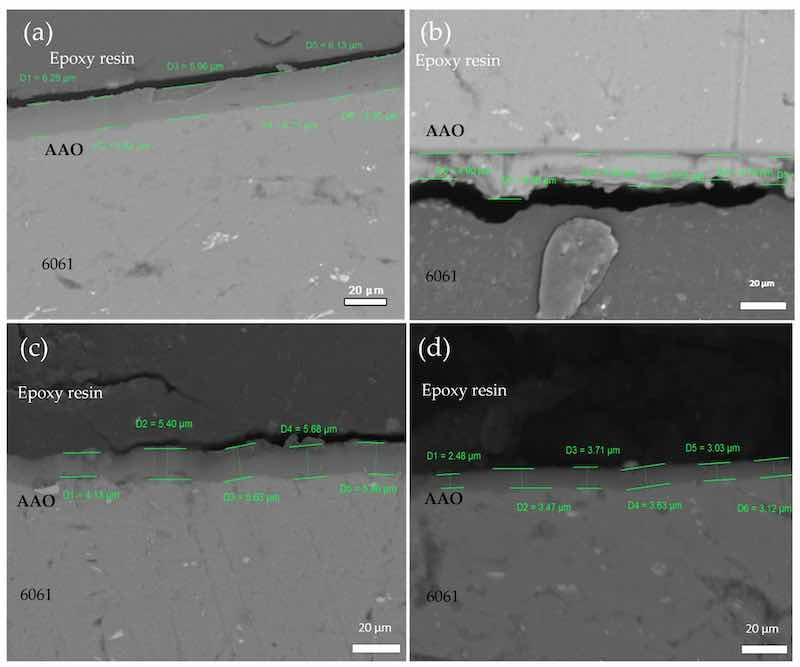
Figure 2. SEM cross-sectional micrograph of AAO of aluminum alloy 6061 at different times and temperatures, using sulfuric acid as the electrolyte: (a) 30-20-SA, (b) 60-20-SA, (c) 30-60-SA, and (d) 60-60-SA, without air injection.

Figure 3. SEM cross-sectional micrograph of AAO of aluminum alloy 6061 at different times and temperatures, using sulfuric acid as the electrolyte: (a) 30-20-CA, (b) 60-20-CA, (c) 30-60-CA, and (d) 60-60-CA, with air injection.
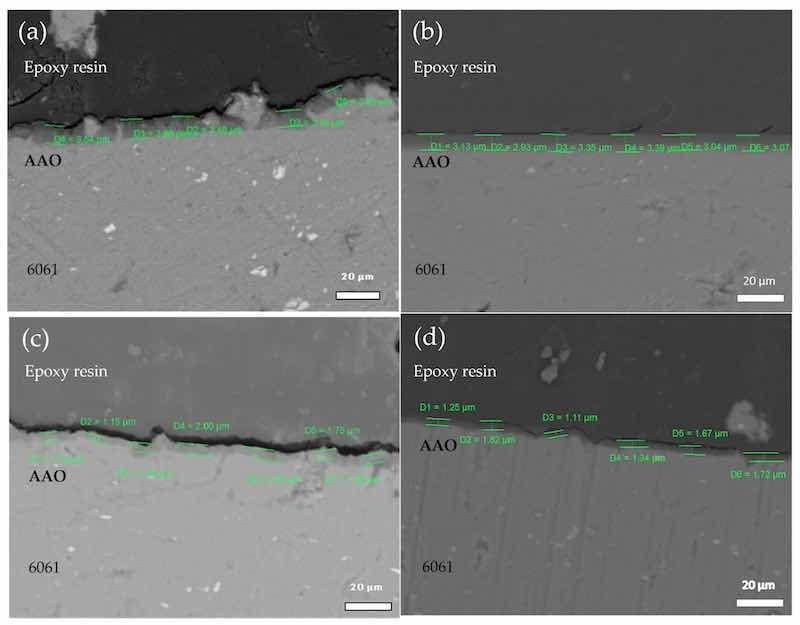
Figure 4. SEM cross-sectional micrograph of AAO of aluminum alloy 6061 at different times and temperatures, using sulfuric acid as the electrolyte: (a) 30-20-CO, (b) 60-20-CO, (c) 30-60-CO, and (d) 60-60-CO, with oxygen injection.
Figure 3a, 30-20-CA y 3b, 60-20-CA show uniform formation of AAO in both micrographs, with lower film thicknesses due to acid dissolution of the anodic layer under temperature conditions of 20 °C and 60 °C with averages of 2.7 and 3.2 μm. Figure 3c (30-60-CA) has an average of 5.5 µm, while Figure 3d (60-60-CA) shows a decrease of 2.7 and 1.4 μm, respectively, with a continuous uniform and stable layer.
Figure 4a (30-20-CO) shows an anodic layer formation of 3.5 µm. In Figure 4b (30-60-CO), the average thickness of the anodized layer is 3.15 µm with uniform morphology. In Figure 4c (60-20-CO), the average thickness visibly decreases to 1.4 µm, maintaining uniformity but with deformation. In Figure 4d (60-60-CO), the film formation is 1.45 µm, which is six times smaller than without air injection, as seen in Figure 2a. The growth rate of the oxide film at high temperatures was much faster than in the low-temperature electrolyte, suggesting that as temperature increased, the driving force to promote longitudinal growth of the oxide film increased with the oxygen bubble overflow and acceleration of the electrochemical reaction.
The relative percentage of the constituent elements of the AAO is shown in Table 5. EDS is a semiquantitative analysis, showing an approach to the chemical composition of the alumina films. A high percentage of sulfur is observed without air injection and low content with air and oxygen injection, due to the formation of sulfates during the anodization process.
Table 5. Relative percentage of elemental energy spectrum of AAO of aluminum alloy 6061.
| Element | Atomic %30-60-SA | Atomic %30-20-CA | Atomic %30-20-CO |
| Carbon | 61.60 | 57.05 | 54.86 |
| Oxygen | 27.57 | 32.67 | 34.30 |
| Silicon | 0.01 | 0.25 | 0.83 |
| Sulfur | 10.17 | 0.52 | 0.33 |
| Aluminum | 0.64 | 9.51 | 9.69 |
| 100 | 100 | 100 |
From a molecular perspective, there may be a better arrangement; the one with better electrochemical behavior might be more compact. When it is observed that the film is neither compact nor porous, it is assumed that surface defects in the growth of the oxide film may be generated due to the presence of nitrogen from the air, which can be absorbed on the surface of the aluminum or trapped during the film’s growth. Sulfur ions might also be absorbed on the aluminum surface. If air is present and the temperature is increased, the growth is limited, and over time the layer may dissolve. With air, increasing the temperature favors growth, but over time it becomes more compact. With oxygen at lower temperatures, the film becomes more uniform.
The AAO formation thicknesses are shown in Figure 5 according to the sample identification, the main conditions under which the anodization process was carried out, and the anodized film thickness obtained under each established parameter. The maximum layer growth limit was 7.4 μm on the base substrate in the sample, corresponding to Figure 1a, 30-20-SA.
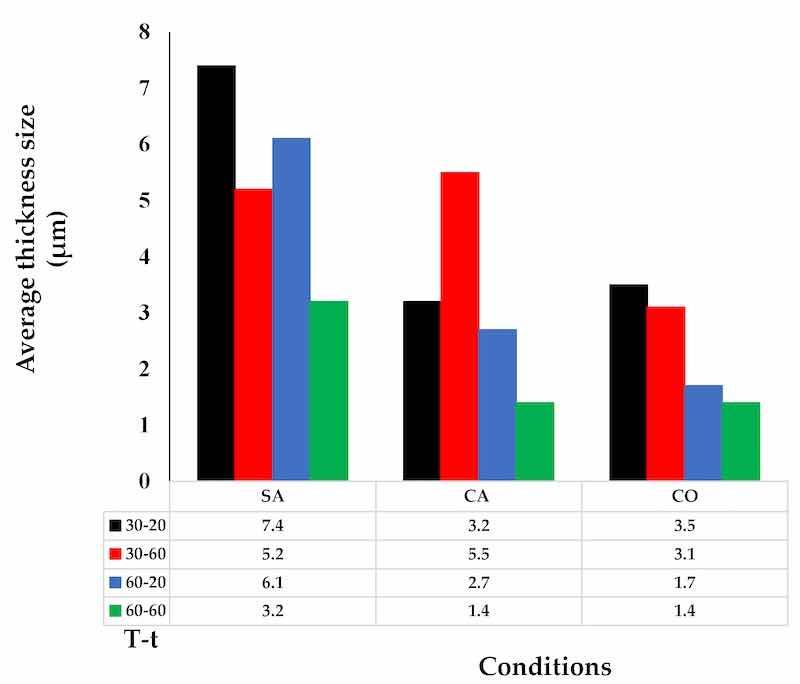
Figure 5. Average formation thickness of AAO by anodization process.
X-ray diffraction patterns (XRD) are shown in Figure 6a without air injection at temperatures of 30 °C and 60 °C, and 20 and 60 min using the grazing incidence technique. It indicates the presence of a broad diffraction peak near 22 °C of the XRD curves, explaining that AAO was not crystalline. The diffraction peaks show the presence of the aluminum phase, indicating that the X-rays penetrated the non-crystalline oxide layer. The diffraction intensities of AAO formation at 30 °C and 60 °C, at 20 and 60 s without air injection, were 2θ = 40°, 47°, 80°, and 85°, corresponding to the Miller indices of the cubic Al2O3 phase (111), (200), (311), and (222), according to the crystallographic chart 01-75-0921. The diffraction intensity of AAO at 60 °C was higher, indicating that AAO in the high-temperature electrolyte could transmit more X-rays than the film prepared at low temperature, further confirming that the oxide layer density decreased as the electrolyte temperature increased. In Figure 6b, with air injection at temperatures of 30 °C and 60 °C, and 20 and 60 min using the grazing incidence technique, the presence of a broad diffraction peak at 30° of the XRD curves is observed. The diffraction peaks 2θ at 40°, 47°, 68°, 80°, and 85° correspond to the Miller indices of the cubic Al2O3 phase (111), (200), (311), (222), respectively, with the crystallographic chart 01-75-0921. The most notable diffraction intensity of AAO at 60 °C was higher, indicating that AAO in the high-temperature electrolyte could transmit more X-rays.
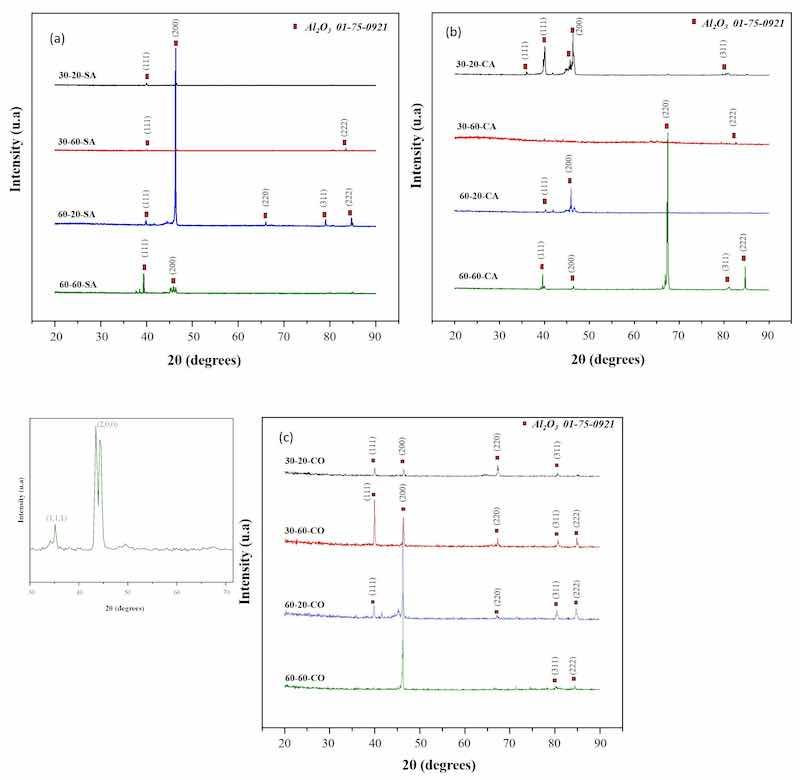
Figure 6. X-ray diffraction pattern of AAO in different temperature and time environments: (a) without air injection, (b) with air injection, and (c) with oxygen injection, using the grazing incidence technique.
In Figure 7a, the potentiodynamic polarization results of the AAO films at temperatures of 30 °C and 60 °C, and 20 and 60 min without air injection are shown. At 30 °C—20 min, activation polarization is present, with the cathodic arm showing stable behavior until reaching the corrosion potential (Ecorr), indicating major susceptibility to corrosion due to an increase in current density, resulting from the low resistance of the sample. At 30 °C—60 min, the corrosion potential (Ecorr) changed to a nobler value with a current density almost a decade lower logarithmically, indicating improved corrosion resistance. In samples 60 °C—20 min and 60 °C—60 min, there is activation polarization, and the current density is an order of magnitude lower than the previous ones. In Figure 6b, the behavior of the anodic film with air injection at 30 °C and 60 °C, and 20 and 60 min is observed. At 30 °C—20 min, a passive layer formation was achieved, showing the cathodic and anodic arms behavior, with a negative Ecorr of −900 mV, indicating active dissolution of aluminum. At 30 °C—60 min, the passive layer formation achieved was stable with a corrosion current density of 2.48 mA/cm2, with the Ecorr moving to more negative values at −1200 mV. At 60 °C—20 min and 60 °C—60 min, passive layer formation with stable behavior was observed, with the Ecorr at −800 mV and −1200 mV, indicating anodic dissolution. Figure 6c shows the anodic film behavior with oxygen injection at 30 °C and 60 °C, and 20 and 60 min. At 30 °C—20 min, the passive layer formation was achieved, with the Ecorr at −1000 mV, showing low corrosion current density (Icorr) behavior at 1.2 mA/cm2, indicating anodic dissolution. At 30 °C—60 min, the passive layer formation was stable, with the Ecorr moving to −1100 mV. At 60 °C—20 min and 60 °C—60 min, stable passive layer formation was observed, with the Ecorr at −1100 mV and −1250 mV, indicating anodic dissolution.
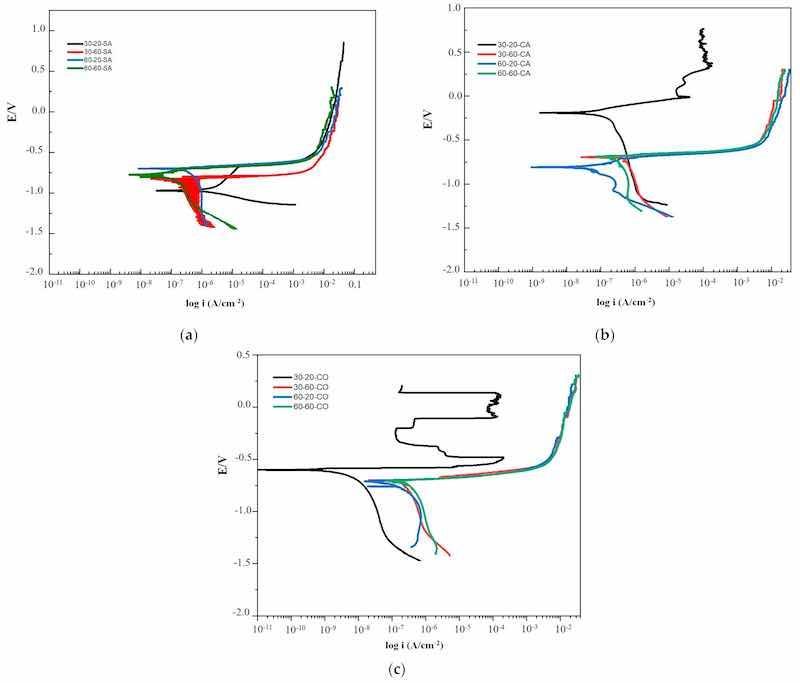
Figure 7. (a) Potentiodynamic polarization curves of aluminum alloy 1061 as a function of processing parameters without air injection; y-axis scale potential vs. current density x-axis scale. (b) Potentiodynamic polarization curves of aluminum alloy 1061 as a function of processing parameters with air injection; y-axis scale potential vs. current density x-axis scale. (c) Potentiodynamic polarization curves of aluminum alloy 1061 as a function of processing parameters with oxygen injection; y-axis scale potential vs. current density x-axis scale.
Therefore, at 30 ° and 60 °C at a time of 20 min, increasing the temperature limits the growth of the layer, produces malformations, and accelerates the kinetics of the reactions.
At 30 °C, from 20 to 60 min, increasing the time degrades the growth of the layer due to the reaction products.
By increasing the residence time and temperature, there is a degradation or dissolution of the layer that forms in the first minutes, which is attributed to the kinetic behavior of the system that is located when increasing the temperature or increasing the residence time.
In Figure 7b, the results of the potentiodynamic polarization of AAO films at temperatures of 30 °C and 60 °C, and durations of 20 and 60 min with air injection are shown. At 30 °C—20 min, a mixed behavior is observed with passivity between 5 × 10−5 and 6 × 10−4 A/cm2 in terms of current density. This phenomenon occurs due to the growth of a stable, compact, and impermeable oxide film, which is the result of the environment to which the sample is exposed. During this process, an additional oxide layer forms that protects the aluminum (passive layer). At 30 °C–60 min, a concentration polarization with similar limiting current values to the previous sample is presented, but with more active values. Regarding 60 °C—20 min, it shows concentration polarization with lower limiting currents by approximately one logarithmic decade, and at 60 °C—60 min, an increase in the limiting current is again observed, showing less corrosion resistance. It is also necessary to discuss this in terms of the structural properties of the anodized film. Therefore, 30° and 60° at 20 min favor the growth of the layer in both cases, obtaining uniform growth, without malformations, which indicates that there is no presence of pores in the film, 30° and 60° at 60 min the size of the layer decreases without malformations, where the electrolyte acts by detaching part of the layer and subsequently becoming passivated again.
In Figure 7c, the results of the potentiodynamic polarization of AAO films at temperatures of 30 °C and 60 °C, and durations of 20 and 60 min with air injection, are shown. At 30 °C—20 min, the test shows the lowest (Icorr) and highest (Ecorr) in this group, demonstrating a passivation phenomenon at 1 × 10−4 A/cm2 and 0.5 V vs. Ag/AgCl over a range of 300 mV, indicating better corrosion resistance. Subsequently, the passive film breaks and re-passivates, possibly due to the formation of anodic sites that are later protected by the passive Al2O3 film, or a pitting corrosion phenomenon might occur. After this, the passive layer attempts to form but is not sufficiently resistant, leading to the generation of pits on the sample as the corrosion potential increases. This is attributed to the oxygen injection during the anodizing process. The curves for 30 °C—60 min, 60 °C—20 min, and 60 °C—60 min show concentration polarization with increments in the limiting current of more than one logarithmic decade, reflecting a sudden decrease in corrosion resistance.
In Table 6, the results of the corrosion rate in mpy are shown. The sample 30-20-CO presents the best corrosion rate, which is due to the fact that materials like aluminum are mainly known for their corrosion resistance. This explains why most samples exhibit a low corrosion rate.
Table 6. Potentiodynamic polarization parameters for the corrosion of anodized aluminum at different pH Values with 3.5 wt.% NaCl, with and without oxygen injection.
| Sample | Icorr (A/cm2) | Ecorr (V) | βa (mV) | βc (mV) | Corrosion Rate MPY (Mils per Year) |
| 30-20-SA | 2.0327 × 10−6 | −0.9891 | 342.65 | −78.84 | 3.7941 × 10−7 |
| 30-60-SA | 9.8137 × 10−8 | −0.90115 | −231.53 | 45.911 | 1.0380 × 10−7 |
| 60-20-SA | 2.3762 × 10−7 | −0.69415 | 23.214 | −284 | 4.4352 × 10−8 |
| 60-60-SA | 2.2916 × 10−8 | −0.77737 | 70.197 | −95.838 | 4.2773 × 10−9 |
| 30-20-CA | 2.9719 × 10−8 | −0.1886 | 62.925 | −79.81 | 5.5471 × 10−9 |
| 30-60-CA | 4.4063 × 10−7 | −0.69703 | 22.375 | −563.12 | 8.2245 × 10−8 |
| 60-20-CA | 2.9470 × 10−8 | −0.80927 | 70.07 | −139.93 | 5.5006 × 10−9 |
| 60-60-CA | 2.1897 × 10−7 | −0.68639 | 24.807 | −401.06 | 4.0871 × 10−8 |
| 30-20-CO | 6.3584 × 10−13 | −0.24103 | −178.71 | 528.16 | 6.7253 × 10−13 |
| 30-60-CO | 3.0776 × 10−7 | −0.72403 | 43.62 | −953.58 | 5.7444 × 10−8 |
| 60-20-CO | 2.3397 × 10−7 | −0.74397 | 44.787 | −466.87 | 4.3671 × 10−8 |
| 60-60-CO | 3.5176 × 10−7 | −0.72688 | 37.626 | −594.76 | 6.5657 × 10−8 |
The results of all the potentiodynamic polarization tests highlight a more corrosion-resistant behavior for the 30 °C—20 min specimen with oxygen injection. This is because it has the least material loss per year and the lowest corrosion current with a higher corrosion potential based on the presented graphs.
There are no changes in the mechanisms, but there is a variation in the activation energy. Nonetheless, when the temperature increases and the time decreases without air, the mechanism changes to a cathodic polarization by concentration. The controls are the same in all the curves under different atmospheres. In the cathodic part, concentration occurs first, and then very close to the corrosion potential, we have mixed polarization. At 30 °C—60 min, the corrosion potential (Ecorr) changed to a more noble value because the layer is more compact as concentration polarization is due to the hydrogen evolution.
4. Discussion of Statistical Analysis
Table 7 shows the real variables of (T) temperature, (t) time, and conditions with air (CO), without air (SA), and with oxygen (CO) scaled, and the response to determine the best anodizing conditions. For the analysis of variance, variables were assigned to designate the response variables: thickness size with Q, grain size with U, and reaction rate with V. For the real variables, i1 and i2 are for low and high time, t1 and t2 are for low and high temperature, and v1, v2, and v3 are for each condition, considering the effects of significant variables and their respective calculated coefficients.
Table 7. General factorial design for aluminum anodizing treatments.
| Sample | Temperature (°C) | Time (min) | Condition | X1 | X2 | X3 | Thickness Size (µm) | Crystallite Size (τ) | Reaction Rate (MPY) |
| 1 | 60 | 60 | SA | 2 | 2 | 1 | 3.2 | 0.08 | 4.28 × 10−9 |
| 2 | 60 | 60 | CA | 2 | 2 | 2 | 1.4 | 0.16 | 4.09 × 10−8 |
| 3 | 30 | 60 | CO | 1 | 2 | 3 | 3.1 | 0.35 | 5.74 × 10−8 |
| 4 | 30 | 20 | CA | 1 | 1 | 2 | 3.2 | 0.04 | 5.55 × 10−9 |
| 5 | 60 | 20 | CA | 2 | 1 | 2 | 2.7 | 1.11 | 5.50 × 10−9 |
| 6 | 60 | 20 | CO | 2 | 1 | 3 | 1.7 | 1.83 | 4.37 × 10−8 |
| 7 | 30 | 60 | CA | 1 | 2 | 2 | 5.5 | 0.63 | 8.22 × 10−8 |
| 8 | 30 | 60 | SA | 1 | 2 | 1 | 5.2 | 1.88 | 1.04 × 10−7 |
| 9 | 60 | 20 | SA | 2 | 1 | 1 | 6.1 | 0.17 | 4.44 × 10−8 |
| 10 | 30 | 20 | CO | 1 | 1 | 3 | 3.5 | 0.94 | 6.73 × 10−13 |
| 11 | 30 | 20 | SA | 1 | 1 | 1 | 7.4 | 0.72 | 3.79 × 10−7 |
| 12 | 60 | 60 | CO | 2 | 2 | 3 | 1.4 | 1.83 | 6.57 × 10−8 |
The resultant was used to predict grain size (3), thickness size (4), and reaction rate (5) by performing a general linear model. The experimental fit shows a certainty of 95.07% (R2 = 0.9507), providing evidence that the mathematical model adequately fits the data.
𝑈 = 0.812 − 0.052𝑡1 + 0.52𝑡2 − 0.010𝑖2 + 0.010𝑖2 − 0.099𝑣1 − 0.037𝑣2 − 0.426𝑣3 (3)
𝑄 = 3.700 + 0.950𝑡1 − 0.950𝑡2 + 0.400𝑖1 − 0.400𝑖2 + 1.775𝑣1 − 0.500𝑣2 − 1.275𝑣3 (4)
𝑉 = 5.026 − 0.402𝑡2 + 0.402 2 + 0.335𝑖1 − 0.035𝑖2 + 0.062𝑣1 − 0.592𝑣2 + 0.658𝑣3 (5)
The results obtained by ANOVA for the response variables Q, U, and V are shown in Table 8. According to the results of this study, it is confirmed that there is significance in the interaction Q−iQ-iQ−i (p > 0.05, p > 0.05, p > 0.05) for grain size and thickness, but for the reaction rate, this significance is not met. For the model (individual effects and main effects), the effect is significant because the p-value is much smaller (p = 0.001, p = 0.001, p = 0.001) than the fixed value of α\alphaα.
Table 8. Analysis of variance (ANOVA) for the variables investigated during the aluminum anodizing process, adjusted to a quadratic model.
| Response Variable | Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F | p |
| Q | Temperature | 10.830 | 1 | 10.830 | 8.85 | 0.021 |
| Q | Time | 1.920 | 1 | 1.920 | 1.57 | 0.521 |
| Q | Condition | 20.105 | 2 | 10.053 | 8.22 | 0.015 |
| Q | Error | 8.565 | 7 | 1.224 | ||
| Q | Total | 41.420 | 11 | |||
| U | Temperature | 0.03203 | 1 | 0.03203 | 0.05 | 0.826 |
| U | Time | 0.00120 | 1 | 0.00120 | 0.00 | 0.966 |
| U | Condition | 1.19152 | 2 | 0.595758 | 0.97 | 0.426 |
| U | Error | 4.31782 | 7 | 0.616831 | ||
| U | Total | 5.54257 | 11 | |||
| V | Temperature | 1.944 | 1 | 1.944 | 0.46 | 0.520 |
| V | Time | 1.345 | 1 | 1.345 | 0.32 | 0.591 |
| V | Condition | 3.164 | 2 | 1.582 | 0.37 | 0.702 |
| V | Error | 29.695 | 7 | 4.242 | ||
| V | Total | 36.148 | 11 |
The p-value of Q as a function of temperature and condition does have an effect on the variable grain size, in contrast to time, which has a value greater than p = 0.005. Regarding the response variable thickness size U, there is no effect as the value is above the estimated p-value; similarly, the response variable reaction rate V presents the same effect with a value greater than p. The levels assigned to the factors were very narrow, so the difference between what happens at one level and another is practically imperceptible for the response variable.
5. Conclusions
From the results of this work, the conclusions regarding the mathematical model for the anodizing process under the variations in the atmosphere and time of expositions and its response to corrosion resistance are as follows.
The results from ANOVA show the response variables for the grain size U, thickness Q, and reaction rate V. These response variables are associated with the time, temperature, and the different atmospheres in good agreement. Thus, the thickness of the layer is correlated to the kinetics of the reaction.
The experiments show a similar linear kinetic behavior in the three systems or atmospheres: they react in the same way, forming the layer uniformly on the surface of the aluminum, but as time passes, there are areas where the aluminum is already occupied, and the reaction passes from linear to parabolic; therefore, the growth of the layer is no longer uniform, and the optimal value for grain size growth was at 30 °C for 20 min with air injection.
Samples obtained through different atmospheres are completely different on the microstructure; this fact causes the anodized layers to present different characteristics and protective properties.
With respect to the corrosion properties, samples present different behavior depending on their structural characteristics. Thus, in the potentiodynamic polarization tests, a more corrosion-resistant behavior can be highlighted for the sample 30-20-CO because it has the lowest material loss per year. It also contains the lowest corrosion current in its process.
At a low temperature of 30 °C, for a short time of 20 min and without air injection, are the optimal values for the linear growth of the anodic layer, where the thickness grows by 7.40 µm. In the potentiodynamic polarization tests, a more corrosion-resistant behavior can be highlighted for the sample 30-20-CO because it has the lowest material loss per year. It also contains the lowest corrosion current in its process, with a higher corrosion potential.
Written by Gabriela Baltierra-Costeira1, Jesús Emilio Camporredondo-Saucedo2, Marco Arturo García-Rentería3, Lázaro Abdiel Falcón-Franco3, Laura Guadalupe Castruita-Ávila2, and Adrián Moisés García-Lara2
- Facultad de Ciencias Químicas, Universidad Autónoma de Coahuila, Blvd. Venustiano Carranza, Saltillo 25000, Coahuila, Mexico
- Facultad de Ingeniería Mecánica y Eléctrica, Universidad Autónoma de Coahuila, Avenida Barranquilla s/n, Colonia Guadalupe, Monclova 25760, Coahuila, Mexico
- Facultad de Metalurgia, Universidad Autónoma de Coahuila, Carr. 57, km 4.5, Monclova 25710, Coahuila, Mexico
Author Contributions: Conceptualization, A.M.G.-L.; Formal Analysis, L.G.C.-Á.; Funding Acquisition, A.M.G.-L.; Project Administration, L.A.F.-F.; Supervision, M.A.G.-R.; Validation, J.E.C.-S.; Writing—Original Draft, G.B.-C.; Writing—Review and Editing, M.A.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding: Universidad Autónoma de Coahuila.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: The data presented in this study are available on request from the corresponding author.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Gaskell, D.R.; Laughlin, D.E. Introduction to the Thermodynamics of Materials, 6th ed.; CRC Press: Boca Raton, FL, USA, 2017.
- Runge, J.M. Anodizing as an Industrial Process. In The Metallurgy of Anodizing Aluminum; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–190.
- Rashid, K.H.; Khadom, A.A.; Mahood, H.B. Aluminum ASA 6061 Anodizing Process by Chromic Acid Using Box–Wilson Central Composite Design: Optimization and Corrosion Tendency. Met. Mater. Int. 2021, 27, 4059–4073.
- Benmohamed, M.; Benmounah, A.; Haddad, A.; Yahi, S. The effect of inhibiting molybdate used in anodizing-conversion treatment to improve corrosion protection of AA2030 aluminum alloy in different steps. J. Eng. Appl. Sci. 2022, 69, 40.
- Kikuchi, T.; Suzuki, Y.; Iwai, M.; Suzuki, R.O. Anodizing Aluminum and Its Alloys in Etidronic Acid to Enhance Their Corrosion Resistance in a Sodium Chloride Solution. J. Electrochem. Soc. 2020, 167, 121502.
- Gower, C.H.R.; O’Brien, S. Electrolyte Containing Sulphuric Acid. UK Patent 290901, 25 October 1927.
- Ono, S.; Saito, M.; Asoh, H. Self-ordering of anodic porous alumina formed in organic acid electrolytes. Electrochim. Acta 2005, 51, 827–833.
- Parkhutik, V.P. Study of Aluminium Anodization in Sulphuric and Chromic Acid Solutions-II. Oxide Morphology and Structure. Electrochim. Acta 1990, 35, 961–966.
- Sulka, G.D.; Stroobants, S.; Moshchalkov, V.; Borghs, G.; Celis, J.-P. Synthesis of Well-Ordered Nanopores by Anodizing Aluminum Foils in Sulfuric Acid. J. Electrochem. Soc. 2002, 149, D97.
- Ono, S.; Asoh, H. A new perspective on pore growth in anodic alumina films. Electrochem. Commun. 2021, 124, 106972.
- Roshani, M.; Rouhaghdam, A.S.; Aliofkhazraei, M.; Astaraee, A.H. Optimization of mechanical properties for pulsed anodizing of aluminum. Surf. Coat. Technol. 2017, 310, 17–24.
- Saffari, H.; Sohrabi, B.; Noori, M.R.; Bahrami, H.R.T. Optimal condition for fabricating superhydrophobic Aluminum surfaces with controlled anodizing processes. Appl. Surf. Sci. 2018, 435, 1322–1328.
- Javaherdashti, R. Microbiologically Influenced Corrosion—An Engineering Insight; Springer: London, UK, 2008.
- Kim, M.; Choi, E.; So, J.; Shin, J.-S.; Chung, C.-W.; Maeng, S.-J.; Yun, J.-Y. Improvement of corrosion properties of plasma in an aluminum alloy 6061-T6 by phytic acid anodization temperature. J. Mater. Res. Technol. 2020, 11, 219–226.
- Hernández, A.; de la Paz Guillón, M.; García, L. La metodología de Taguchi en el control estadístico de la Calidad. Filosofia 2015, 23, 65–83.
- Cullity, B.D. Elements of X-ray Diffraction, 3rd ed.; Addison-Wesley: Reading, MA, USA, 1956.
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.



































