Deoxidizing solutions are widely used as a subsequent treatment after etching aluminum. However, the inherent properties of deoxidizing solutions allow for a wide range of applications from enhancing the activity of the substrate to removing chemical film.
This paper will delve into the basic mechanisms of deoxidizer chemistry, what applications deoxidizer solutions can be used for and solution maintenance for these applications. This is a comprehensive view of all roles that deoxidizer solutions can be used in plating.
Introduction
Most of us call ourselves aluminum finishers. As many of us know how difficult it can be to anodize aluminum, providing the most optimal surface for anodization may be just as difficult and overlooked. In many settings, surface preparation may consist of some sort of alkaline cleaning or de-greasing followed by an etchant and then desmut. Each of these is very important to the anodization process; but this paper will focus deoxidizing of aluminum, not just desmutting.
Desmut is the act of removing excess alloyed metals from the surface from aluminum after etching. Desmutting can be done in any mineral inorganic acid such as hydrochloric, sulfuric, nitric, etc. Even some old time anodizers would desmut in the anodize tank before adding current! However; these solutions offer so much more potential than removing complexed alloys from the surface.
Deoxidizing may sound more sophisticated, in application it is, but it can provide a better surface for anodizing compared to regular desmutting. Deoxidizing removes aluminum oxide off the substrate layer of aluminum via redox reactions, which normal mineral acids cannot achieve. You can always desmut aluminum, but you may not always deoxidize aluminum prior to processing.
In this paper, redox reactions are defined and discussed, for they make up the basic chemistry of deoxidizing. The types of deoxidizers; whether it is chromium, iron, or fluoride based, each variety of chemicals has an application. It is important to know when or when not to deoxidize, considering many people may not deoxidize at all. It is also important to know which deoxidizing solution is needed for a specific application. Using the wrong solution can ruin parts. Lastly, salvaging parts and repairing any defective coating from types 1, 2, 3 anodizing and chemical conversion will be discussed and recommendations given.
Target Audience
- Any NADCAP/Commercial anodizer operator
- Any NADCAP/Commercial plater operator
- Chemical Engineers, Chemists, Metallurgist, anyone who does contract review in your facility
- Researchers whom develop chemicals or systems to help aid in surface preparation of aluminum.
The Basic Chemistry of Deoxidization and Redox Reactions
Aluminum is an interesting element because of its inherent nature to oxidize readily in contact with oxygen. Since aluminum is not found naturally, it is found as bauxite, it tends to change phases to protect itself from corrosion.4 This process called passivation, happens when bare aluminum is in contact with oxygen. A very thin layer of aluminum oxide forms and creates a non-active surface, electrochemically.6 This thin layer of oxide is what we seek in anodizing, but it is not of a sufficient thickness to serve our applications. Thus, we need to remove it with a solution called a deoxidizer.

Above: A picture of freshly mined Bauxite, the precursor to aluminum processing. Source: https://en.wikipedia.org/wiki/Bauxite
Aluminum corrosion is commonly encountered when performing chemical process operations involving surface finishing, predominantly in preparation from anodizing, plating or paint.9 Aluminum readily protects itself in stable conditions, around pH of 4.5-8.5, by chemically creating aluminum oxide with the surrounding oxygen.9 Deoxidizers intentionally exceed this pH range for cleaning, metal removal and subsequent smut removal. Deoxidation is a method which is used in the preparation of anodizing which helps remove oxygen from the surface of the aluminum.6 In contrast, antioxidants are used for stabilization such as in preparation of food.6 Deoxidizing is achieved by adding a chemical such as ferric sulfate to a desmutting solution to help aid in the removal of oxygen on the surface of the aluminum.
Deoxidizers are able to work because of the transferring of electrons called Redox reactions. Redox is short for reduction-oxidation reaction. The definition of Redox is a chemical reaction in which the oxidation states of atoms are changed.7 Any such reaction involves both a reduction process and a complementary oxidation process, two key components involved with electron transfer process.7 The chemical component, called a species, may have an electron stripped from it or added on. In the case for when it is stripped, the species is said to have been oxidized and when the species gains an electron, it has been reduced. It can be simplified below:7
- Oxidation: is the loss of electrons or an increase in the oxidation state by a molecule, atom or ion.
- Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom or ion.
 Above: This is a visual interpretation of oxidation and reduction. Source: https://en.wikipedia.org/wiki/Redox
Above: This is a visual interpretation of oxidation and reduction. Source: https://en.wikipedia.org/wiki/Redox
The processes of oxidation and reduction occur simultaneously and cannot happen independently of one another, like in acid and base reactions.7 Each reaction occurs in what is called a half-reaction. Two half-reactions will always occur simultaneously to form a whole reaction. When solving a Redox reaction, the gained or lost electrons are typically included explicitly in order that the half-reaction is balanced with respect to electric charge.7
“Though sufficient for many purposes, these general descriptions are not precisely correct. Although oxidation and reduction properly refer to a change in oxidation state - the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is best described as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classified as “redox” even though no electron transfer occurs, such as those involving covalent bonds.7”
In a Redox reaction, the transfer of electrons happens between two chemical species. The reducing species always transfers electrons to the oxidation species. It is important to know the terms given to each chemical species in the Redox reaction. One who is new to chemistry or this type of reaction may have trouble with all the convoluted terms and expressions given to these species. An easy way to remember which species the oxidizer and reducer is by using this memory aid: OILRIG. “Oxidation Is Loss of electrons, Reduction Is Gain of electrons.”7 In the reaction the reductant or reducing agent loses electrons and is oxidized and the oxidant or oxidizing agent gains electrons and is reduced.7
Chemical species that can oxidize other substances are said to be oxidative or oxidizing are known as oxidizing agents, oxidants or oxidizers.7 Once this oxidizer removes electrons from another substance it has then reduced itself and can be also called an electron acceptor. Oxygen is the quintessential oxidizer.7 Oxidizers by nature have higher oxidation states and some examples are hydrogen peroxide, oxygen, fluorine, chlorine, and nitric acid.
Chemical species that can reduce other substances causing them to gain electrons are said to be reductive or reducing and are known as reducing agents, reductants, or reducers.7 The reducing agents will oxidize itself and transfer electrons to another chemical species in the Redox reaction. Since this chemical species will donate electrons, it can also be called an electron donor. Reductants are very diverse in chemistry and some examples are lithium, sodium, iron, hydrogen gas, platinum.7
This basis of whether a redox reaction will happen is based off standard potential. Each half reaction has a standard potential, which is equal to the potential difference or voltage at equilibrium under standard conditions of an electrochemical cell. 7 In this reaction the cathode will be reduced and the anode will be oxidized. So therefore, when anodizing you’re oxidizing the hardware and during plating you’re reducing the hardware. The electrode potential of each half-reaction is known as the reduction potential or potential when the half-reaction takes place at a cathode.7 The reduction potential measures the tendency of the oxidizing agent to be reduced. The oxidation potential is a measure of the tendency of the reducing agent to be oxidized, but does not represent the physical potential at an electrode.7 An easier way to interpret it would be the more positive the value, the higher chance it will be reduced. The more negative the value the higher chance it will be oxidized. The further the number is away from zero, the more “forceful” the compound will be to undergo transformation and become unstable.
There are many examples of Redox reactions that currently are happening all around us, inside of us and are waiting to happen based on cell potential. A couple of real world examples of Redox reactions included rusting of metal, aluminum oxide formation, pitting of metal, anodizing, cellular respiration, photosynthesis and many more. Since these reactions may be difficult to visualize, a breakdown of how rust occurs will be provided in the following paragraphs:

This is a visual interpretation of oxidation and reduction of iron to form rust. Source: https://www.wiley.com/college/boyer/0470003790/reviews/redox/redox.htm
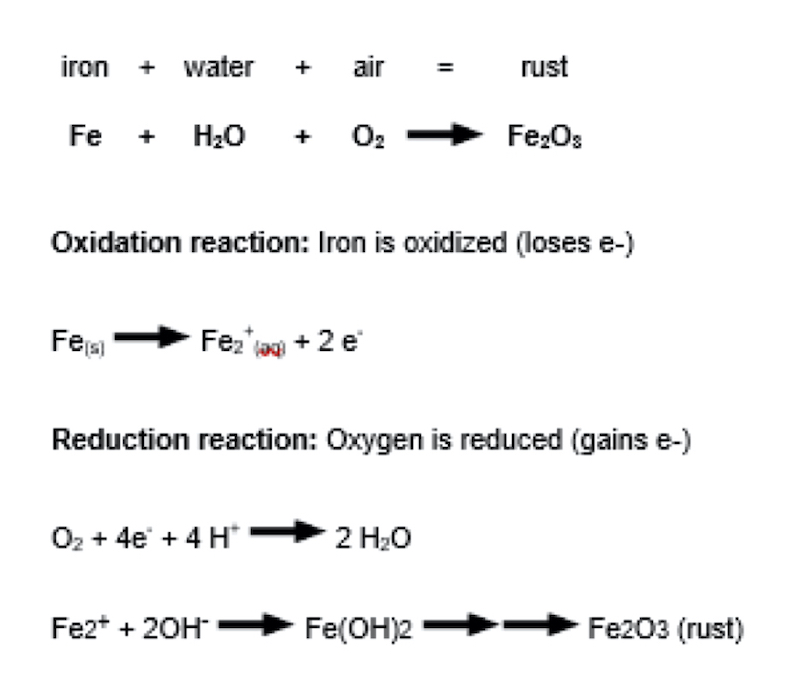
This is a visual interpretation of oxidation and reduction of iron to form rust. Source: https://www.wiley.com/college/boyer/0470003790/reviews/redox/redox.htm
Above is the well-known sight of rust. Rust is a flakey, brittle, burnt-orange colored substance that forms when iron and oxygen are left exposed to one another, especially if the rust gets wet. What may be unknown is that rust does not form on iron, it is iron converted to iron oxide. Rust is a form of oxidized iron.10
The reaction above explains how the Redox reaction occurs. First, iron in the presence of moisture (H20) will lose electrons becoming a positively charge ion in water. Those electrons are then used to reduce the oxygen dissolved in water. The Fe+2 ions reach with the OH- ions in water to produce iron hydroxide, which will dry in several steps to produce rust. Notice that the Redox reaction needs water; which explains why a moist environment speeds up the rusting process10. For us platers and anodizers, this is the exact test we use to see if free iron is present after passivation. In a high humidity chamber, accelerated rusting is forcing a steel part to show if iron is present on the surface.
Deoxidizing of aluminum can only be successful if there is aluminum oxide present on the part or is there is a chemical species willing to donate or accept electrons in the solution. In our facility, we use a chemical by the name of LNC Deoxidizer by Chemetall. This is an approved Deoxidizer for Boeing and many other NADCAP subscribers. The key constituents in the chemical solution are ferric sulfate and nitric acid. Since Ferric is in the +3 states, it can be the oxidizing agent and reduce the Aluminum oxide on the surface of the aluminum. Nitric acid is very important because of its inherent oxidizing abilities. This helps speed up the reaction to lower time spent in the deoxidizer. Once the thin layer of aluminum oxide is gone, bare aluminum is the substrate left and has been “activated.” Activation means that the bare aluminum is looking to donate electrons to maintain equilibrium. This is why deoxidizing is right before anodization or plating.
Types of Deoxidizers
As you might know, there is an infinite amount of ways to chemically formulate your deoxidizer to achieve the results needed. However, most approved deoxidizers are usually made up of a few strong acids combined with some type of chemical to help drive the Redox reaction. The most commonly used acid solutions are nitric-, sulfuric-, or chromic- acid based. Nitric and sulfuric are usually interchangeable or paired, so I will only discuss nitric-based, as they are more common. Each of these acids is quite powerful and operators in each plant should have extensive safety training and proper PPE. In the next few paragraphs, multiple types of deoxidizers will be explained and how to formulate them to achieve optimal results. They will be broken down by types of acid used.
The first of the two “non-etching” deoxidizers are Nitric-acid based deoxidizers. These solutions are some of the most common deoxidizers used today. Nitric acid is commonly a choice because of its ability to slowly attack aluminum and because of its autocatalytic abilities as an oxidizer. Nitric acid deoxidizers are usually light-duty deoxidizers and are principally employed as a desmut. “However, it is important to remember that when running any metals high in magnesium, nitric acid based deoxidizers are most effective. This is because nitric acid will remove magnesium oxide scale, which can often interfere with caustic etching or other surface treatments.11” Nitric acid based deoxidizers will produce satin type finishes while not removing the finished metal shine. Nitric acid base deoxidizers are useful for salvaging parts. Nitric acid Is known to “open” the pore structure of anodic films which allow for easier stripping of anodize. Nitric acid based deoxidizers can be used before any subsequent processing such as paint, chemical conversion, anodizing, etc.
An example of a typical nitric acid base deoxidizer has these components:
- Nitric Acid 15-25%
- Iron in the form of Ferric Sulfate: 60-70%
- Ammonium Bifluoride: 5-10lbs/100gal
An example of how nitric acid reacts with bare aluminum; the aluminum oxides and alloying element smut-layer compounds are made soluble by being converted into their respective nitrate forms.9
- Al2O3 + 6HNO3 => 2AL(NO3)3 + 3H2O
- Al(OH)3 + 3HNO3 => Al(NO3)3 + 3H2O
As the base aluminum further reacts, producing more soluble aluminum nitrate and hydrogen gas:
- 2Al + 3HNO3 + 3H2O => AL(NO3)3 + Al(OH)3 + 3H2(g)
These are all components beneficial to the deoxidizer tank. Source: http://www.pfonline.com/articles/aluminum-surface-finishing-corrosion-causes-and-troubleshooting
This solution is an effective deoxidizer/desmut which can be used at room temperature and no fume exhaust is needed. The immersion time for this solution is 1-5 minutes. The proper rinsing after a solution like this should be city water, but low in chlorides and other TDS raising ions. Triple rinsing is the most effective way of removing the ferric solution. These deoxidizers are typically the most environmentally friendly since there is no chromium involved. These deoxidizers can have tanks made of stainless steel 316, polypropylene, or have some sort of Kynar liner.
The second of the “non-etching” deoxidizers are chromium based deoxidizers. Chromic based deoxidizers are the gentlest of all the deoxidizers. These deoxidizers will passivate bare aluminum and will tend to confer a passivating action on any other solution to which it is added.11 Chromic based deoxidizers are popular in the removal of heat treat films and to prepare aluminum alloys for zinc – immersion plating, chromic acid anodizing, painting and other chemical treatments. It helps eliminate surface irregularities caused by oxide inclusions or embedded buffing particles and does not, in general, removes nearly as much metal as caustic soda, while it leaves the surface clean and semi-bright.11 Chromium based deoxidizers work best with tanks made from 18-8 stabilized, stainless steel-clad material, lead lined, Kynar liner or High-density polypropylene. (Note: Do NOT used PVC as chromium based compounds will attack the welds!).
An example of a typical Chromium based Deoxidizer has these components:
- Chromic Acid: 5-10%
- Nitric/Sulfuric Acid: 7.5 – 15%
- Ammonium Bifluoride: 5lbs/100gal
- Rest: Deionized Water.
This solution can be used at room temperature with immersion times between 5-10 minutes. As most of you all know chromium is a carcinogenic substance which most European countries are trying to eliminate. These deoxidizers will be replaced with fluoride/nitric based deoxidizers. Be careful not to rinse in too clean of water as this will deactivate the surface!
An aggressive deoxidizer, which can be used on castings, shot-peened, or blasted parts are hydrofluoric acid based deoxidizers. These deoxidizers will give a light etch and a matte finish. These deoxidizers are very effective against casting alloys because of the fluoride’s ability to dissolve silica. Silica is, for the most part, a chemically inert element which is used to make glass and in aluminum castings. Fluoride – acid based deoxidizers should be used very carefully and operators should be fully trained in self-first-aid if they are using these types of solutions. It only takes about 25 in2 of a splash on the skin to kill a grown male with hydrofluoric acid. This acid does not burn initially when it contacts your skin, which makes it even more deadly because of your unawareness of contact. Please use caution and train your personnel thoroughly before using this solution.
An example of a typical fluoride based deoxidizer has these components:
- Hydrofluoric Acid: 10%
- Nitric Acid: 10%
- Rest: Deionized water
An example of how hydrofluoric acid reacts with aluminum.9
The hydrogen fluoride component in this type of deoxidizer has numerous mechanisms that produce soluble aluminum fluoride, hydrogen gas, and aluminum hydroxide.
- 2Al + 6HF => 2AlF3 + 3H2(g)
- 8Al +6HNO3 + 24HF => 8AlF3 + 3N2O(g) + 15H2O
Some aluminum hydroxide reacts, further producing a duo-anionic solid
- 2Al +2HNO3 + 2H2O => 2Al(OH)3 + 2NO(g)
- 2Al(OH)3 + AlF3 => 3ALOF(s) + 3H2O
Hydrogen fluoride is consumed with the production of soluble ammonium nitrate
- AL + 3HF + HNO3 => AlF3 + NH4NO3
One of the interesting aspects in the listing of this reaction is the large amount of hydrogen fluoride being consumed. This means that as a desmut tank ages; hydrogen fluoride must be replenished daily to keep consistent strength.9 This solution should be at room temperature, unless otherwise authorized by an OEM. Typical immersion time for this solution is 4-6 minutes. If this solution is heated, the proper fume extraction should be implemented and the fumes should be expelled to current state law and regulations.
Deoxidizer solutions are prone to anionic (chlorides) and cationic (copper)contaminations, which directly results in pitting. For this reason, the copper concentration should be checked monthly and deionized water should only be used for solution make-ups and rinsing. Failure to capture these limits can scrap hardware or pose for costly rework.9
Deoxidizer Applications
Deoxidizers can be used in a variety of ways to help prepare aluminum surfaces for subsequent processing and help salvage parts that have failed final inspection. Salvaging parts will be discussed later in this paper.
Deoxidizers should be employed for the following conditions:11
- Cleaning with little or no etching prior to the application of a suitable chemical conversion coating prior to the application of organic coatings or electroplated coatings.
- As a desmut bath or neutralizing rinse after alkaline etching
- Removal of oxide, mill scale, corrosion products, heat treatment scale, or welding fluxes.
- Matt etching or bright etching for decorative effects.
- Deep selective etching for patterns in relief.
- Very deep etching for chemical milling or contouring.
- Etching to increase the surface area of electrolytic capacitor anodes.
- Undercut etching to give a mechanical key for PTFE application.
- Electrolytic etching for lithographic printing purposes.
- Heavy duty deoxidizing for casting
- Light duty deoxidizing
- No-etch call out
Some of these topics will be further discussed in the following section.
At the company I work for, Aerotech Processing Solutions, we process mostly machined parts which are for the aerospace industry. A lot of the parts are complex and they have tight tolerances and usually call out “do not etch” on the purchase order/contract. Since we process many parts for the aerospace industry, the alloys of choice are 2000 and 7000 series alloys. These alloys are alloyed with copper, zinc, magnesium, etc. These intermetallic inclusions, especially in 7000 series which yield superior strength and corrosion resistance, will react with the atmosphere almost readily. Most of the time these parts do not come in oiled or protected, so they have oxide film build up or the beginning of pit formation. Since you cannot etch these parts, but the surface need to be cleaned up and extended immersion time of a light duty deoxidizer is employed to the process. This treatment will remove any oxidation films or localized pitting before anodize or plating can take place. Obviously light duty deoxidizers can be used to remove any kind of smut after alkaline etching.

Left: Part that came in for chemical conversion. Notice the corrosion in the center of the circle. Right a part that was chemically treated with a nitric-iron deoxidizer. This deoxidizer is a light duty deoxidizer which does not removed large amount of metal. Notice how the oxidation spots are removed.
For aerospace OEM’s function is not all that matters when processing hardware. Sometimes the part is not painted or dyed and will be left anodized in the plane or helicopter. This means there is an emphasis on cosmetics and surface finish. When you alkaline etch a part it is common to increase the surface roughness by at least 3x and take the shine away from the aluminum. Most aerospace machine shops finish their aluminum surfaces to a Ra of 32. This is usually a requirement per many prints. When you caustic etch, you can easily reach RA of 50 to 60! Which means you will never pass final inspection and you have non conforming product. What the deoxidizer allows in a controllable way to clean up the surface appearance without metal removal.
Deoxidizers can also be used to fix welding fluxes and heat treat scale. Some parts may come in that were previously welded and non destructive tested. These welding marks can contain cavities and crevices that will hold electrolytic solution and could damage the part post processing. Using a heavy duty deoxidizer such as one with nitric and sulfuric acid can help reduce the weld cavities and make them smoother. This will allow the solution to not be entrapped post processing. Heat treatment scale is very difficult to remove and can prevent steels from becoming passive. This could be a problem because if they are not passive then the material will eventually rust, resulting in field failure. Hydrochloric acid can be used to remove heat treat scale. This scale can be removed within 2-3 minutes using a 10% hydrochloric acid solution. You can use this solution as a pretreatment to alkaline cleaning then subsequent passivation.
For parts that are shot peened, blasted, or casted a heavy duty deoxidizer is the only type that can clean up the surface appearance. Fluoride is the key to this deoxidizing method. As was discussed earlier in this paper, the fluoride ion is heavily used and intensifies the etching process. Parts that are shot peened or blasted will need a heavy etch to smooth out all the rough surfaces associated with the process. A normal caustic etch could do this, but again it is much more difficult to control the metal removal. Since parts that are shot peened are usually aerospace parts, etching wouldn’t be allowed so the only alternative would be to use a heavy duty deoxidizer with fluoride.
Casted alloys are a completely different process when it comes to surface preparation. Since these alloys are high in silica and other uncommon metals, the common practice of clean, etch deoxidize cannot be used. Etching castings with alkaline solutions will cause severe cosmetic problems and paint adhesion failures. This is because of the high proportionality of alloyed material, silica, on the surface of the aluminum. The high density of metallic inclusions on the surface will cause voids in the anodize substrate and this ultimately will cause paint failure. The other bonus of using fluoride in deoxidizers is to give a frosty matte finish. Depending on the customer needs, a frosty matte finish is cosmetically appealing and also gives a good finish for dye operations. Most decorative finishes will use deoxidizers with fluorides present in them.
Deoxidizing aluminum also comes with its challenges and there are times where you would not want to use a deoxidizer. There are two reasons why you wouldn’t use a deoxidizer; one if the part is going in for NDT or if the material is very high in copper, well past 2024. Copper is a known contaminant of deoxidizers and too much copper may cause pitting. This can be seen on some casting alloys, 2219, 2319, and 2011. These 2000 series aluminum have high ductility and are great for welding.8 In the case of NDT, if smut get deposited on a part before NDT from a dirt deoxidizer solution it can mask discontinuities and/or cause pitting. Deoxidizers that are old will have suspended smut in the solution, you can verify this by checking the corners of your deoxidizer tank. This situation of having a tank which is too high in impurities can have the same effect when you are chemical filming. A loose smut layer can cause catastrophic paint adhesion failure.
Maintaining Your Deoxidizer
In this section the facility, quality, manufacturing control and good general practices will be discussed. Some of these practices you can relate to other tanks and preparations in the plating/anodizing industry. Facility and manufacturing controls will discuss how the tanks and actual equipment should be constructed and how the personnel should handle parts which will be deoxidized.
Quality control will discuss the frequency and what you should be testing to maximize the effect your deoxidizer has on hardware. Lastly, good general practices are things to keep in mind when processing parts that will involve deoxidizers. These are the practices that we use at our facility and is indoctrinated in Aerotech’s Process Control Document.
Facility controls are very important when you are running a plating line. These suggestions are given to you to help get the most out of your plating line and keep the process under control. If a process can fluctuate rapidly, your final product will follow suit. Consistency is key with special processing and this can be obtained the more controls you have for your process. Any control with a measurable value should be calibrated in your system. This accuracy will help with keeping consistency in your process. It is important to make sure that the material of your tanks is acid resistant, including the welds of the tank. Over time, especially if you have more aggressive acids, the welds can fail and may develop a leak. This is hazardous and expensive to clean up.
Any tank that is heated should have some sort of temperature indicator readily visible to the operator. This helps the operator gauge immersion times and will also prevent over etching. A rule of thumb to remember is the reaction rate doubles roughly for every 10°F you go up or down. If you remove .001 mils every 30 minutes in your deoxidizer at 85°F, if you raised the temperature to 95°F your new etch rate would be around .002 mils every 30 minutes. Keeping constant temperatures will help keep consistent metal removal.
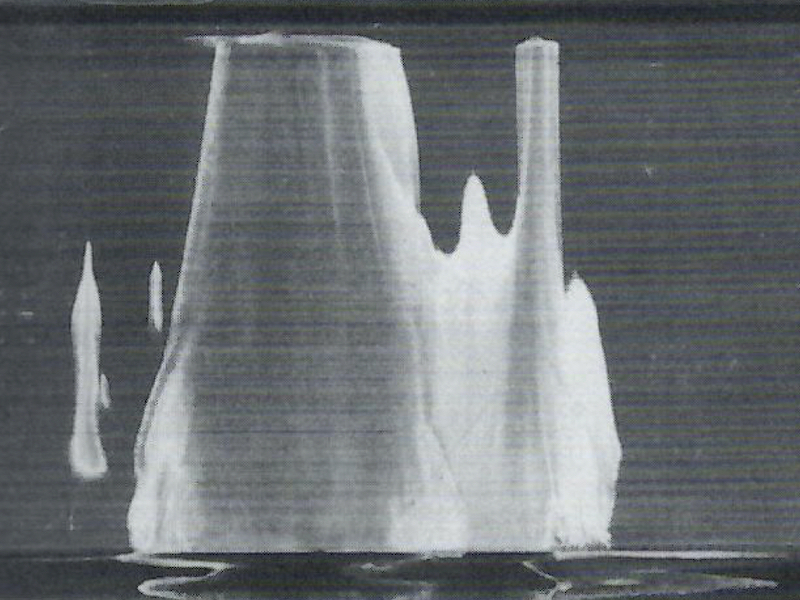
Above: An example of severe etch staining resulting from local overheating Source: Brace, Arthur. (2000) The Technology of Anodizing Aluminum. Modena, Italy: Interall S.r.l.
Solution agitation is a must have for a plating line. Concentration and temperature gradients will form under laminar conditions. The more turbulence added to the tank the more uniform the gradients will be. This is important if you are running big loads of parts which may be 4 – 5 feet from each other in the tank. The part on the upper left may receive much more removal then the part on the bottom right if you do not have uniformity in your tanks. The picture above is an example of etch staining from severe overheating.
Fume exhaust or some type of scrubbing vents may be mandatory depending on what chemicals you use and what state you live in. If you are heating any acid, or using chromic or hydrofluoric acids it should be a requirement to have this installed. Fumes are highly corrosive and can cause major damage if they encounter mucus membranes of the operator. If you are using chromium, a scrubber should be used and the wash down should reenter the tank. This will keep any chromium from leaving the building and it will help replenish the tank throughout the day, just be sure to use deionized water!
Manufacturing controls can be implemented to help operators limit their contact with the hardware. Minimizing the handling of pretreated metal parts is a must. The sodium chloride found in the oils and sweat of the operator will eventually pit the aluminum if contact is made for enough time or if it is frequent enough. The operators should handle the parts with gloves such that the outside surface of the gloves is kept clean, dry, lint and powder free. The real key to keeping contamination at a minimum is always having a clean supply of gloves at your operator’s station to constantly keep changing them out. Over time gloves will get contaminated with oil from hardware that was freshly machined. White or light-colored gloves are usually the best gloves to choose because they can reveal dark colored contamination on the hardware. It is also advised that the gloves do not contain any silicone or other substance which will be detrimental to material adhesion.
Quality control is just as important as the other two controls. Quality control will keep the solution, equipment, and operators from changing behaviors and delivering varied results. Solution control is a must if you are a NADCAP shop or if you are looking for higher end commercial work. One of the main tasks would be to monitor the process and examine the parts frequently to make sure everything in your process control document is being met. The chemical solutions should be analyzed weekly to ensure proper maintenance of composition, stability, and etch rates. Other testing may be required by the OEM the parts are for or from the technical data sheet of the product itself. There are 6 tests which should be assigned to the deoxidizer tank to make sure the tank is running properly, they are:
- Monthly temperature readings: using a calibrated thermometer against your in-tank probe is a proper way to monitor the fluctuations in your equipment. If there is a major difference between the two instruments, you should use the one that is calibrated the most recent and adjust your temperature accordingly.
- Rinse water: you should always use deionized water. Chlorides and other heavy metals can pit a surface that has been freshly activated from deoxidizing. I would recommend the TDs be between 50-300 ppm. If the rinse water is too clean, below 50 TDS, then the water will passivate the part and prevent surface conversion as well.
- Etch Rate: The only way to tell the effectiveness of your deoxidizer is through an etch rate. You’re etch rate will determine your immersion times and will help you gauge the strength of your deoxidizer. Depending on what specification you are maintaining your deoxidizer to, our metal removal limits should be controlled. This can be controlled via temperature or concentration of the constituents. Most specifications will allow for a max metal removal limit and this is based off the strongest and hottest the solution will go. If your etch rate over the upper limit, either lower the temperature, add water or decant the solution.
- Solutions Analysis: Depending on what solution you use or what the specification says, solution analysis should be done on each constituent at least once a week. Most deoxidizers can have their concentration evaluated via acid/base titrations and these titrations are easily found in textbooks. Using this structure, an add schedule can be made to keep the tanks as consistent as possible. Remember chemical consistency produces consistent products.
- Examination of parts: This examination should be done by the operator after any deoxidizing step. What the operator should do is look for any smut that is still present on the part after deoxidizing. If the maximum immersion time has been reached and the smut is still present, then a decant or addition should be made to the tank.
- Intergranular Attack/End grain Pitting: This test is a little more advanced and will need to be sent to a testing laboratory. If your shop isn’t NADCAP approved you probably won’t be doing this test. However, the deoxidizer shall not cause end grain pitting more than 0.001 inches or intergranular attack more than 0.0002 inches of depth. If your solution does cause either one of these two phenomena then the solution is scrap and will need to be remade. However, this is rare and unless the solution is overly strong or hot, you should not run into this problem.
Lastly, general good practices (GGP’s) should always be employed at the processing facility. Some of these GGPs are self-explanatory and I’m sure most facilities are using them, but it is always good to reinforce what we already know. Here are some GGP’s:
- Parts should always be completely immersed in the tank. This means before having parts enter the line, make sure each solution is properly filled.
- Parts shouldn’t be allowed to dry between tanks, this can cause water stains which will show up after anodizing/plating.
- When racking, the position and shape of the part should be taken in account to make sure that the circulation of solution is touching the whole surface area of the part. You should also keep conscious of air pockets and blind holes which can entrap solution.
- Keep the solution free of excessive oils, scum, floating debris, and other foreign material.
- Deoxidizers may accumulate sludge requiring occasional removal and this sludge should never encounter any part. Desludging requires decanting the settled bath into a clean tank and then removing the sludge from the bottom of the tank. When this is done, you can return the solution to the tank, agitate and re-test.
Salvaging Parts
This area of processing is somewhere no processing house wants to be. However, it is inevitable that you will find yourself at times with borderline scrap parts if you cannot strip and re-plate. There are a few options, which I will explain, that the deoxidizer can provide which aid in stripping of anodize. I am not too familiar with plating so I will not discuss stripping of plating coatings. Aluminum oxide forms 1⁄2 above the datum plane and 1⁄2 below the datum plane. So remember when you strip off the coating and you re-plate you need to add back double to reach your dimensional tolerance. The next section will discuss how to add anodize to already existing aluminum oxide and the best method to strip anodize and/or chemical film.
When applying type 2 or especially type 3 anodize you may encounter a time where you have to go back in to add more thickness, after the part has been completed. At this point the part is usually sealed, for type 2, or the pores are completely hydrated, for type 3. This means that there is a barrier layer that will make it difficult to reapply anodize because the resistance is high and the surface is not active. To re-activate the surface, it is recommended that a nitric acid pretreatment of 8-12% for 10 minutes or a light duty deoxidizer for about 5 - 10 minutes prior to re-plating. This pre-treatment will reduce time for re-plating and also help prevent burning due to the high resistance of anodic coating.
When stripping off anodic coating, having a solution that’s controllable is most important. Using caustic etch is reliable and fast if you can control the solution. Temperature and concentration are the most important parameters when etching. Using an etch rate formula you will be able to control how much material you remove. Aluminum oxide will etch off faster than bare aluminum so always try to etch for half the time to see how much material was removed. Deoxidizers come into play when you have aged anodic coating. A soak in the deoxidizer will help soften up this coating. It is not required, but it will prevent differential etching of the anodize. Anodize does not seal uniformly, you will have areas of the part that will seal better than others due to the pore structure. This will then cause differential etching. The deoxidizer will help “break” the seal before the aluminum oxide layer gets attacked.
The other way you can strip anodize is using a chromic-phosphoric solution. This solution, when made fresh, will only strip anodic coating and will leave the shine on aluminum. The solution is about 2% chromic acid by weight and 3.5 % phosphoric acid by volume and heated to 212°F.1 The immersion time should be no longer than 7 minutes and it is imperative that the solution does not have more than 5 grams per liter of dissolved coating in it.1 This will cause Intergranular attack to the base metal. An easy way to keep track of the stripped coating is to weigh your parts before and after stripping. This should be done in a lab with small parts that have very tight tolerances. If the part is bigger than a 2-L beaker, I would stick to the caustic soda method.
Conclusion
Deoxidizers offer so much more than one might initially suspect. The variety of solutions that may be used and the applications for these solutions is really endless. Keeping the quality and facility controls on your deoxidizer solution is a must to get the full benefit of having the solution on your plating line. Always remember that the deoxidizer can be used to help bail you out of a situation and/or provide a clean activated surface for subsequent plating or anodizing operations.
Peter Totaro is Vice President of Operations at Aerotech Processing Solutions, a Nadcap-certified, special processing facility serving the aerospace, defense, and other high tech industries. Totaro is our chemist and engineer, and is currently a PhD candidate in Chemical Engineering.
References:
1) ASTM Standard B137-95, 2014, “Standard Test Method for Measurement of Coating Mass Per Unit Area on Anodically Coated Aluminum,’ ASTM International, West Conshohocken, Pa, 2014, DOI: 10.1520/B0137-95R14. www.ASTM.com.
2) Brace, Arthur. (2000) The Technology of Anodizing Aluminum. Modena, Italy: Interall S.r.l.
3) Ferroecoblast.com/r_d/sand_blasting_and_shot_blasting/
4) https://en.wikipedia.org/wiki/Aluminium
5) https://en.wikipedia.org/wiki/Bauxite
6) https://en.wikipedia.org/wiki/Deoxidization
7) https://en.wikipedia.org/wiki/Redox
8)chttps://materialsdata.nist.gov/dspace/xmlui/bitstream/handle/11115/173/Aluminum%20and% 20Aluminum%20Alloys%20Davis.pdf?sequence=3
9) http://www.pfonline.com/articles/aluminum-surface-finishing-corrosion-causes-and- troubleshooting
10) https://www.wiley.com/college/boyer/0470003790/reviews/redox/redox.htm
11) Sheasby, P.G. (2001) The Surface Treatment And Finishing Of Aluminum And Its Alloys. Trowbridge, Wilts BA14 8RB, UK: Redwood Books.
12) www.aerospacemanufacturinganddesgin.com/article/amd-0310-laser-sintered-titanium-eos-shellabear/
13) www.progressivesurface.com/aerospace.htm



































