The anodizing industry faces increasing pressure. As production cycles shorten, anodizing suppliers are required to deliver faster.
 Dr. JuhlThe type II anodizing process takes on average 2 minutes per micron (0.04 mils) formed oxide. That means a 20 micron (0.8 mils) oxide layer takes 40 minutes to anodize. Pulse anodizing can reduce this process time by half and thereby give your company a competitive edge. Read this article to learn more and sign up for the free webinar about pulse anodizing at AnodizingSchool.com.
Dr. JuhlThe type II anodizing process takes on average 2 minutes per micron (0.04 mils) formed oxide. That means a 20 micron (0.8 mils) oxide layer takes 40 minutes to anodize. Pulse anodizing can reduce this process time by half and thereby give your company a competitive edge. Read this article to learn more and sign up for the free webinar about pulse anodizing at AnodizingSchool.com.
Using square pulse anodizing means to have a higher average current density for the total process and thereby reduce the process time. However, higher current density will often lead to problems such as burning of parts, especially for copper-containing aluminum alloys. Pulsating between two values of current density, a high period and a low period, will give the aluminum surface time to relax after the high current period. The time for these periods should be seconds to minutes to make sure that the surface will recover properly. This recovery effect is the reason that pulse anodizing was developed the first place [1].
The Recovery Effect
The advantage of anodizing in pulses can be explained by the recovery effect. To keep it simple the whole electrical system is considered to be the formed oxide layer. This system has to obey Ohm´s law, so when aluminum oxide is formed the thickening of this will increase the resistance of the system.
Ohm´s law: V = R x I (Voltage equals Resistance times Current)
When a high voltage E1 is applied, the responding current will reach a steady level i1 after a certain time, as seen in Figure 1. During this period t1, the resistance R1 and the current i2 will reach a level corresponding to the forming voltage E1. When the voltage is suddenly lowered to E2, the current density will decrease drastically to a very small value as seen in period t2. This low value of the current, sometimes in the range of µA, corresponds to the very high resistance R1 the oxide layer formed during the high voltage period). The electrical field across this interface is very low and therefore will the formation of oxide be almost zero (c – d). The main reaction in this period will be the chemical dissolution of oxide. This period is called the recovery period and is the period where the surface will be prepared for another round of high current density. In this period the resistance R2 will be stabilized corresponding to the resistance of the system for this lower value of voltage.
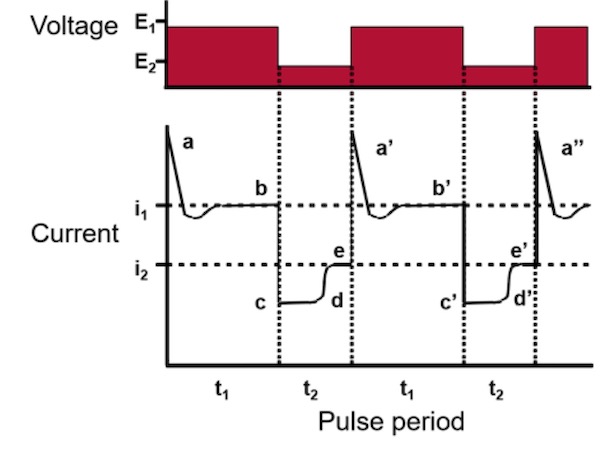
The time for this recovery depends on many factors such as alloying elements, concentration of the electrolyte, temperature of the electrolyte and the value of E. The thickness of the interfacial layer has become thinner, hereby increasing the electrical field across it because of a lower resistance of the total system. This will lead to an increase in the field-assisted dissolution and formation will take over, increasing the in the current to a value of i2, corresponding to the resistance of the reduced thickness of the oxide layer. Then it is time for increase the voltage again, so the current density increases and by this start the formation of more aluminum oxide – important here is, as always to remember that it is the current which forms the oxide layer and not the voltage. By using this pulse anodizing method, the average current density can be raised to 3 – 4 ASF or even more and by this decreasing total process time for the anodizing step.
Case Study “Energy optimization in the anodizing process – an electrolytic process” sponsored by the Danish Power Distribution
During my PhD about pulse anodizing of extruded and cast aluminum alloys I found that pulse anodizing not only increases productivity but found also indications for energy savings [2]. The company Hydro (formerly SAPA) hired me to investigate the opportunities of pulse anodizing to save energy and we got funding from the Danish Power Distribution. In this project I found that by using slow square-formed pulses, the process time could be decreased up to 50 percent, and, at the same time, the total energy consumption in the anodizing tank was decreased up to 30 percent [3].
The aluminum alloy used was EN AW 6063 alloy. The chart shown in Figure 2 gives an idea of the tremendous reduction in time forming the same thickness of the oxide layer. The process parameters were conventional DC anodizing at 18 ASF, and three different average values of current densities used for the slow square-pulse anodizing.
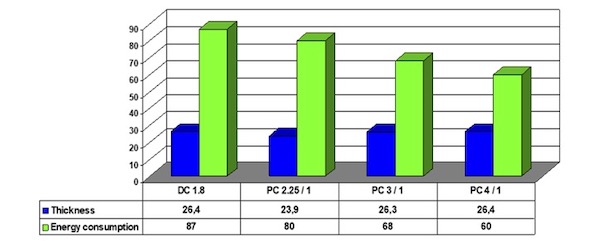
The first one was run by 22.5 ASF in the high-current period and 10 ASF in the low period. The second one used 30 ASF in the high- current period and 10 ASF in the low, and the last one using 40 ASF and 10 ASF. The process time was calculated to give a thickness of the oxide layer of 0.8 mils (the resultant thicknesses were a little higher – figure in microns), and the pulse time was 120 seconds for the high current and 30 seconds for the low current. The voltage drop was logged every second during the anodizing process in different places on the load. The energy consumption is calculated from the logged voltage drops and the current running through the system. The energy consumption was then calculated in MWh/m3, which gives an idea of the amount of energy used per micron-formed oxide layer. The conventional DC anodizing process uses 87 MWh/m3 compared to the slow square-pulse anodizing process with 40 ASF in 120 seconds and 10 ASF in 30 seconds with a total process time of 18 minutes.
ROI Increasing Revenue
The following example shows how pulse anodizing increases ROI. If the volumes of the tanks are 800 gal, with three anodizing tanks each connected to a DC rectifier of 25V/4000A. The sealing process is hot sealing. To make it easier to calculate, the loads are all 0.8 mil sulfuric acid anodizing type II, class 1 coatings, which take 40 minutes each (1 load per rectifier per hour). This gives a production of 24 loads per shift. The total area on each load is 20 m2.
The average value of each load is set to $100, and the average number of workdays in a year is 260 days. With 48 loads a day, the total revenue for a year is $1,248. Changing the total production to slow square-pulse anodizing will increase the total revenue per year to $2,782. The increase in revenue is due to the reduction in the anodizing time (18 minutes vs. 40 minutes) for the 40 ASF and 10 ASF current density process, giving a total of 107 loads a day.
Investment
Most of the anodizing job shops run their rectifiers to the maximum limit. To be able to use pulses, three new rectifiers should be purchased to get the total increase in revenue. At the same time, new customers or increasing orders from current customers are needed to fill the free slots and thereby double the number of loads.
Most anodizers will probably buy one new pulse rectifier to begin with, giving roughly a 33 percent increase in total loads. So instead of 107 loads per day it will only be 67 loads, compared with 48 loads for the conventional DC anodizing line. The total revenue per year is then $1,742, giving an increase of $494 per year with one new rectifier. The price of rectifiers varies a lot depending on their abilities, the way they are built and what supplier is chosen. The company would need to buy one new rectifier with a 30 V/8,000 A specification.
Depending on the cooling capacity of the existing cooling system and the agitation system already installed in the tank that is changed to pulse anodizing, there could be a need for investment in these two systems as well. Again, depending on the condition of the contact points, busbar and rack design, there could be a need for upgrading of these parts, too. In other words, there are two different investment scenarios. The first scenario is a small investment with a new rectifier and a bigger cooling system. The other scenario is a big investment with a new rectifier, cooling equipment, contact and racks, and a new agitation system. The last investment scenario will probably only be interesting if the anodizing process is fully switched from conventional DC anodizing to slow square-pulse anodizing.
Scenario 1 has an estimated cost of $40,000 for the rectifier [4] and $40,000 for the new cooling system that can be used for the other anodizing tanks too, giving a total cost of $80,000. Scenario 2 has the same cost for the rectifier and then upgrading of the rest of the equipment mentioned above. The upgrade is estimated at $150,000, giving a total cost of $190,000. The ROI in both scenarios is less than a year, which must be considered to be a valid investment.
A lot of the anodizing shops in the U.S. are already using 24-30-V rectifiers, so it could be sufficient to add a process controller to those rectifiers to be able to pulse anodize instead of buying a new rectifier. To do this, an inspection will be required at the anodizing job shop to see which investments are needed to upgrade the existing DC rectifier to pulse rectifier. The price of a rectifier is mostly depending on the voltage and not so much on the current, so this scenario will have an even faster ROI.
Energy considerations
Energy is not a part of the above calculation. As measured in the Danish project, there is not only a decrease in time but also a 30 percent decrease in the energy consumption during the slow square-pulse anodizing.
According to the Energy Information Administration [5], energy costs approximately 5 cents per kWh. The energy decreases by 27 MWh/m3 in going from conventional DC anodizing to slow square-pulse anodizing. This will give a small decrease in energy costs when replacing one DC rectifier of $4,914 per year. Another big source of energy consumption in an anodizing line is the heated tanks. When the anodizing tanks can handle double the number of loads, the same number of parts can be processed in half the time.
If the anodizer goes for one pulse rectifier without having more customer parts, there will still be an energy savings. Forty-eight conventional DC anodizing loads are processed in 16 hours (= 2 shifts). Replacing one of the DC rectifiers with a pulse rectifier would process the same number of loads (48) in 12 hours instead of 16 hours. By this it would be possible to shut down the whole anodizing line approximately one day earlier a week, hereby reducing the energy used to keep the temperature up in the heated tanks.
As shown in the above calculations, it is clear that an increase in productivity would pay the investment in the new pulse technology. Moreover, as energy is an increasingly scarce resource, the energy savings will benefit the ROI, too, and give the anodizing shop a competitive edge – especially since anodizing is often seen as an energy-heavy process.
The calculation is for the pulse pattern 40 ASF in 120 seconds and 10 ASF in 30 seconds. The values are suggestions to start with, and some anodizing lines will need a lower current density in the high period. Every anodizing line will have its own optimum values, so to get the best results some test loads should be taken into account before running optimal. To learn more about pulse anodizing, check out the free webinar at AnodizingSchool.com.
Dr. Anne Deacon Juhl, owner of AluConsult and Anodizing School, and an industry expert in aluminum anodizing. Contact her at info@anodizingschool.com
References
- Yokoyama, K., Konno, H., Takahashi, H. and Nagayama M., Anodic oxidation of aluminum utilizing current recovery effect , AES, 2nd. International Symposium on Pulse Plating, Rosemont, Ill., USA, Oct. 6-7, 1981.
- Juhl, A. Deacon, Pulse Anodizing of Extruded and Cast Aluminium Alloys , Ph.D. thesis, Inst. of Manufacturing Engineering, The Technical University of Denmark, July, 1999.
- Juhl, A. Deacon, Burfelt, K. and Weldingh, P., A new approach to pulse anodizing Decreasing energy consumption Increasing productivity , presented at the 15th Annual International Anodizing Conference & Exposition, October 2 5, 2006, Toronto, Canada.
- American Plating Power, www.usplating.com
- http://www.eia.doe.gov/neic/brochure/electricity/electricity.html



































