The uncertain economic trends, and environmental compliance regulations, are issues we encounter on a continual basis. These are actual challenges with regard to the cost of doing business.
There are many subsections or portions of a business that are due a good review. By using a practical approach to confirm various operating parameters, better ways of doing things are identified. The benefits include improved quality, better production throughput, reduced costs, and hopefully easing the demand for the waste treatment system, to name a few.
It is especially in these challenging times that we need to determine how to improve the overall operation, focusing on throughput, economy, and safety. As an example, let us consider the first step in most process cycles, cleaning. This is a big step, in fact, the most important step in any line operation.
The Cleaner
 Belt Skimmer. Courtesy Abanaki Oil SkimmerThe concentrate may be an emulsifying or displacement type. About 90% of soak cleaners are alkaline, having a pH from 9-14, depending primarily on base metals processed. With an emulsifying cleaner, oily soils are held or encapsulated at the operating temperature. On cooling, there may be some release of these oils. A displacement type cleaner tends to displace oils from the substrate surface. This occurs continually at the cleaner operating temperature or in association with a solution temperature reduction in an overflow sump.
Belt Skimmer. Courtesy Abanaki Oil SkimmerThe concentrate may be an emulsifying or displacement type. About 90% of soak cleaners are alkaline, having a pH from 9-14, depending primarily on base metals processed. With an emulsifying cleaner, oily soils are held or encapsulated at the operating temperature. On cooling, there may be some release of these oils. A displacement type cleaner tends to displace oils from the substrate surface. This occurs continually at the cleaner operating temperature or in association with a solution temperature reduction in an overflow sump.
Since most oils are less dense than water, they will float on the bath surface. In either case, released oily soils can be removed by mechanical devices and filters. By regularly removing such soils as contaminants, the service life of the cleaner is extended. Removing the soils has the effect of maintaining a higher degree of cleaning efficiency and improving the cleaner bath service life. It is also important to maintain the cleaner concentration in the suggested range. This is accomplished by maintenance additions and analysis. From an operational standpoint, related benefits of continually removing oily soils include:
- Reduction in rejects due to poor cleaning
- Less downtime to dump and make a new cleaner bath.
- Savings on cleaner products concentrate
- Reduced demand on the operating waste treatment system
- Less interruption to the production cycle.
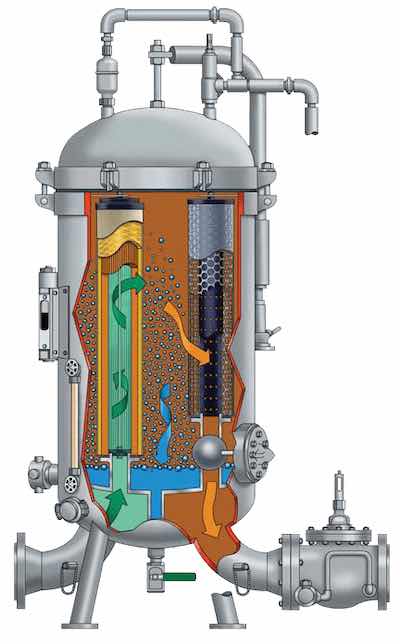 Coalescer. Courtesy Precision Filtration ProductsFrom a cleaning application on steel, a really good, alkaline emulsion cleaner may hold 8-10% of oil at the point of saturation, ready to dump. On the other hand, displacement cleaning continually removes the oily soils. With displacement cleaning, oil belt, coalescer, or disc skimming, overflow weir with side tank are examples of available equipment.
Coalescer. Courtesy Precision Filtration ProductsFrom a cleaning application on steel, a really good, alkaline emulsion cleaner may hold 8-10% of oil at the point of saturation, ready to dump. On the other hand, displacement cleaning continually removes the oily soils. With displacement cleaning, oil belt, coalescer, or disc skimming, overflow weir with side tank are examples of available equipment.
We have three types of cleaners with which to work. These are powder, liquid, and additives. Powder cleanershave been a staple of the industry for many decades. They continue to be heavily used and offer a wide variety of dependable formulations, tackling all types of cleaning demands. Liquid cleaners are formulated as equivalents to powders, offering some additional benefits. Some of these benefits include significantly less sludging (a plus when considering F-006 regulations and the cost to ship sludge) and easier, safer makeup. As well, the concentrate is assured of being 100% blended throughout. The liquid can be metered, and the solution is continually analyzed by conductivity measurement (SPC and NADCAP benefits). For larger volume users, liquid cleaners may be supplied in returnable totes, eliminating the disposal of empty drums.
A third cleaning option is the use of additives in combination with generic liquid caustic soda (usually 50% strength). This is primarily applied to the cleaning of steel. An additive concentrate, typically liquid, is added in a specific ratio with liquid caustic soda in the soak cleaner and a different ratio for both in the electrocleaner.
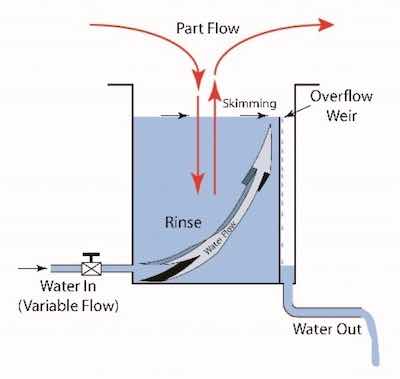 Cleaner Tank with Overflow WeirBranching off the traditional stand-alone soak and electrocleaners, we may consider a dual-functioning cleaner. A combination soak and electrocleaner can be set up in one tank or in separate process tanks where a rinse in between can be eliminated. The benefits here are: simplifying the inventory of products, omitting a rinse (conservation of water), and having a dual function in one process tank (saving on heating and maintenance). In fact, the previous description of additive and liquid caustic soda can fall into this cost savings category, offering extra savings. The use of generic liquid caustic soda reduces the cost of chemicals used.
Cleaner Tank with Overflow WeirBranching off the traditional stand-alone soak and electrocleaners, we may consider a dual-functioning cleaner. A combination soak and electrocleaner can be set up in one tank or in separate process tanks where a rinse in between can be eliminated. The benefits here are: simplifying the inventory of products, omitting a rinse (conservation of water), and having a dual function in one process tank (saving on heating and maintenance). In fact, the previous description of additive and liquid caustic soda can fall into this cost savings category, offering extra savings. The use of generic liquid caustic soda reduces the cost of chemicals used.
This discussion of cleaner types may find a logical fit. Or perhaps the system in place is right. However, the examples given may give enlightenment to implementing improvements. Remember that for any consideration of change, due diligence in the form of proper prescreening is important.
Analysis
It cannot be overemphasized how important analytical control is and should be for a cleaner. The most common analysis has been the neutralization titration with a standard acid solution to an indicator-induced solution color change. It is hard to improve on this method, as most cleaners contain complex wetter and surfactant systems. It can be very difficult and cost-prohibitive to try analyzing on such a broad or complete level. The operation continues with cleaner bath analysis by titration (or test kit), maintenance adds, and rolling along until dump time. Should the operator be fixed to dumping the cleaner after a determined quantity of cleaner has already been added? This is sort of reminiscent of dumping after the maintenance additions are double the initial makeup quantity. Are we certain the cleaner bath is actually “shot”? Maybe it still has 25% or some other viable figure of service life left. This is where some simple yet effective additional analysis can help to determine the dump cycle more sensibly. Here are some suggestions. The following are examples to determine cleaner bath service life as practical tips.
Specific Gravity: As the used cleaner ages, oils, grease, and related soils & particulates will build up in the working solution. These contaminants will contribute to raising the cleaner bath’s specific gravity.
Method
- Obtain a sample of the freshly prepared cleaner bath. There should have been no parts as yet processed in the cleaner.
- Adjust the solution to a selected standard, such as 77°F (25°C).
- Measure the specific gravity either by weight or hydrometer method (be consistent.
- Repeat this procedure for the cleaner bath on a routine basis. The concentration of the cleaner should be equivalent to the initial makeup.
- Record the data. For example, graph the specific gravity versus cleaner bath age.
The point at which the cleaner has been determined to be dumped will coincide with the specific gravity. For production schedule purposes and optimum cleaning, the shutdown required to dump the cleaner can be reasonably predicted.
Oil Splitting: This procedure determines the volume of emulsified oils that are present in the working cleaner solution. Adding cleaner concentrate to the bath may not always deliver the required quantities of wetting agents and surfactants, along with other hard surface cleaning agents. Additionally, the saturation level of emulsified oils would also require dumping the cleaner. This test serves a dual purpose. It supplements the titration procedure to determine how much cleaner maintenance addition is required. It also helps to determine when actually is optimum to dump the cleaner.
Method
- Measure 180 milliliters of the cleaner bath into a clean 250 milliliter Corning type beaker. Allow the solution to cool.
- Slowly add 20 milliliters of 10% Sulfuric Acid solution while the solution is stirring. Be Careful! Wear proper safety equipment!
- Heat the solution with continuous stirring to 150°-160°F (66°-71°C) for 10-15 minutes, permitting the released oils to be displaced.
- Stop stirring, and rapidly pour the solution to the 100-milliliter mark into a clean graduate cylinder.
- Let the solution stand and cool.
- Observe for oil and grease separation.
- Take a final measurement of oils and solids that have split out. Direct read.
Based on the chemistry of the specific cleaner, its detergency system, and particular cleaning requirements, the emulsifying capability during aging can be tracked. The point for dumping may coincide with reduced cleaning efficiency due to increasing emulsified oils. Extra adds of the cleaner concentrate may confirm no practical cleaning improvement.
Settling: This is a rapid test to track the buildup of organic and solid soils in the cleaner as it ages.
Method
- Pour a sample of the hot cleaner bath into a 100-milliliter graduate cylinder to the mark.
- Allow the contents to settle.
- Measure the displaced soils on the bottom of the graduate cylinder and on the surface. The direct reading is the actual percentage of displaced soils. As the cleaner ages, these contaminants build up. Repeat the test on the ready-to-dump cleaner. Use this data to interpret a graph of displaced soils versus the age of cleaner.
Cleaning Test: This procedure incorporates a practical cleaning test to determine the degree of contamination and cleaning ability of the working cleaner.
Method A Performance Soak Cleaner
- Select representative parts that will fit into an appropriate size glass beaker.
- Pour a sample of working cleaner into the beaker. Maintain temperature as per operating parameters.
- Immerse the part in the cleaner with mild agitation for 60 seconds.
- Remove the part and rinse for 60 seconds in clean, cold running water.
- Remove from the rinse and observe for water breaks for a period of 30-60 seconds. If there are no water breaks, continue with the next step. If there are water breaks, refer to information that follows this cycle.
- Immerse the water break-free part from step #5 in 5% of either Hydrochloric or
- Rinse the acid-dipped part in clean, cold running water for 60 seconds. Remove and observe for water breaks.
- If there are no water breaks, tilt the part at a 60-degree angle, allowing the water to drain downward by gravity.
Cleanliness is confirmed by the absence of water breaks after the acid dip. Additionally, steel parts will begin to flash rust. This can be observed after 1-2 minutes.
If, upon analysis, the addition of cleaner concentrate has been made and there is still a cleaning problem, the previous test would be an appropriate evaluation to determine if more cleaner concentrate, beyond the titration analysis, is required.
Referring to Step #5, if water breaks occur, add 10-20% of the initial charge of cleaner concentrate to the bath. Repeat the evaluation as necessary until the water breaks have been eliminated. Determine how much cleaner is required. Depending on the amount, it may be economical and practical to replace the current cleaner bath with new makeup.
Method B Performance Soak Cleaner
Refer to Method A. immerse a clean steel panel in the soak cleaner (a clean hull cell panel is satisfactory). Water breaks can be interpreted as suspended oils and grease re-depositing on the panel. The specific cleaner bath may be deficient in detergency and deflocculants or approaching its dump cycle. The recommendations as per the previous discussion apply to determine appropriate additions or follow-up action.
Method C Electrocleaning Test
This evaluation helps to determine the effect of contaminants in the working solution. We are specifically referring to oils and grease, metallic smuts, hexavalent Chromium, chloride, low caustic, or concentrate buffer and inhibitors out of balance.
- Prepare an electrocleaning cell using a square poly pro vessel or a hull cell. Insert a cleaned steel panel as the cathode (it may need to be cut into form).
- Pour the electrocleaner solution into the cell. Maintain at the desired operating temperature.
- Insert a cleaned steel panel as the anode by placing directly opposite the cathode.
- Anodically electroclean at the selected and current density for the specified time.
- Remove the panel and follow steps 3-7 in Method A.
- Wipe the panel with a clean white paper towel, detecting any deposited smuts. Similarly, examine the cathode panel.
Observation may indicate specific problems. Corrective methods are also suggested.
- Brown or black corrosive spots (fine dots). This may be chloride contamination due to the drag in of hydrochloric acid, especially in a double cleaning cycle. Chlorine gas bubbles form on the anode surface, forming corrosive pits. Increase the electrocleaner concentration. Caustic and silicate are effective inhibitors for chloride contamination. Improve rinsing to minimize chloride drag. If permissible, switch to a non-chloride acid.
- Water breaks on the anode panel. Oil and grease depositing on the panel. Improve the soak cleaning, improve rinsing to minimize drag in of soak cleaner, use oil separation (as described) in the soak cleaner, switch to a higher detergency electrocleaner, or a combination soak and electrocleaner.
- Electrocleaner solution is yellow colored, whereas normally it is not. Additionally, there may be a marked decrease in foaming during the electrocleaning cycle. This would be due to hexavalent chromium contamination. Add a chrome reducing agent to correct the problem or change to an electrocleaner blended with the chrome reducer. Minimize the source of hexavalent chromium contamination, such as stripping rack tips or reject parts.
- In the electrocleaning test, a rusty brown film on the anode. The electrocleaner concentration is low. Specifically, the caustic (sodium hydroxide) portion of the concentrate product is low. Adjust electrocleaner concentration or replace concentrate with one blended with a higher caustic concentration.
- Brown or black smuts that rub off or are soluble in acid would indicate metallic smuts depositing on the anode panel. Adjust electrocleaner concentration to boost the level of complexor or inhibitor.
For cleaner evaluations, instrumental analysis can also be helpful. For example, by using atomic absorption, the cleaner can be analyzed for various metal contaminants. For example, a soak cleaner may tolerate 600-800 ppm zinc, 200-300 ppm nickel, and 100-200 ppm copper. An electrocleaner may tolerate ten times the concentration of these metals. However, the sensitivity to Chromium can be as low as 25 ppm.
By comparison to other chemistries used in metal finishing, cleaners are relatively inexpensive. However, being the first step in most cycles, the effect of satisfactory cleaning is of utmost importance. By utilizing easy yet effective analysis tests that supplement the titration, the cleaner bath service life can be optimized, thus economizing the general operation.
Stephen F. Rudy, CEF, is president of Chem Analytic and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.



































