Manufacturers are exploring moving to new critical product cleaning processes that use either organic solvents or water blended with a bunch of chemicals, including organic chemicals.
We’re going to get to know a little about molecules. Why bother? Perhaps you are reading product literature that asserts superlative cleaning performance of a cleaning agent. Understanding the chemical name (not just the mystery brand name) makes it easier to understand how the cleaning agent works.
 Barbara and Edward Kanegsberg Chemical structure determines chemical function. You probably wouldn’t buy a new car or a new 3D printer sight unseen. You’d want to look at it to understand a bit about construction, quality of workmanship, and functionality. The same is true for cleaning agents. Because cleaning agents are made up of chemicals, getting comfy with molecular structure will help you appreciate the way cleaning agents function. Understanding chemistry will help you manufacture more effectively and economically.
Barbara and Edward Kanegsberg Chemical structure determines chemical function. You probably wouldn’t buy a new car or a new 3D printer sight unseen. You’d want to look at it to understand a bit about construction, quality of workmanship, and functionality. The same is true for cleaning agents. Because cleaning agents are made up of chemicals, getting comfy with molecular structure will help you appreciate the way cleaning agents function. Understanding chemistry will help you manufacture more effectively and economically.
Alcohols, modified alcohols, acetone, and isoparaffin hydrocarbons are referred to as organic chemicals or organic solvents, or simply solvents. What do we mean by organic chemicals? When we teach cleaning courses, we find that some people think of “organic” in the context of agricultural practices. Organic solvents do not mean chemicals that are cage-free, free-range, or non-GMO. Understanding the system for naming organic chemicals helps remove the mystery associated with cleaning agents.
The physical and chemical nature of the molecules that make up cleaning agents determines how they function, including the cleaning process and the cleaning equipment.
Organic solvents are made up of one or more molecules that contain the element carbon. In contrast to the ionic bonds in table salt (NaCl), carbon forms strong bonds, called covalent bonds, with up to four other atoms. The physical and chemical nature of the molecules that make up cleaning agents determines how they function, including the cleaning process and the cleaning equipment.
Alkanes
A hydrocarbon contains two elements: carbon and hydrogen. An alkane is a saturated hydrocarbon. While carbon can be connected to another atom by two or even three bonds, saturated hydrocarbons contain only single bonds. Paraffin is an older name for saturated alkane.
The name of an alkane is usually written with the suffix “ane” and with a prefix that reflects the number of carbon atoms (Table 1). So, an alkane with four carbons is butane.
Table 1: Organic chemical prefixes
Prefix Number of carbons
Meth 1
Eth 2
Prop 3
But 4
Pent 5
Hex 6
Dec 10
Eicos 20
Prefixes for numbers greater than four are derived from the Greek word for that number. If the chain is longer than three, there can be isomers that have the same number of atoms but with different shapes. Different shapes result in different chemical and physical properties. Professor Darren Williams at Sam Houston State University likes to say that there is not a huge difference in the shape of a basketball and a football, but they sure bounce differently. The alkane isomers can be linear or branched. Linear alkanes are called normal, frequently abbreviated as n. Branched alkanes are called iso. For example, butane has two isomers, n-butane, and isobutane.
 Butane C4H10 and Iso-Butane C4H10Another name for isobutane is 2-methylpropane. This is more descriptive and is the preferred name based on the nomenclature of the International Union of Pure and Applied Chemistry (IUPAC). The name reflects that a single-carbon methyl group (CH3) is a side chain attached at position “2” (the middle) of a 3-carbon (or propyl) chain. The IUPAC names become important for alkane isomers of greater than four carbons when there could be one or more side chains attached at different carbons in the parent chain. The number of isomers increases rapidly as the number of carbons increases. Decane, with 10 carbons, has 75 isomers; eicosane, with 20 carbons, has over 300,000 isomers.
Butane C4H10 and Iso-Butane C4H10Another name for isobutane is 2-methylpropane. This is more descriptive and is the preferred name based on the nomenclature of the International Union of Pure and Applied Chemistry (IUPAC). The name reflects that a single-carbon methyl group (CH3) is a side chain attached at position “2” (the middle) of a 3-carbon (or propyl) chain. The IUPAC names become important for alkane isomers of greater than four carbons when there could be one or more side chains attached at different carbons in the parent chain. The number of isomers increases rapidly as the number of carbons increases. Decane, with 10 carbons, has 75 isomers; eicosane, with 20 carbons, has over 300,000 isomers.
Isoparaffins (Paraffin Hydrocarbons)
Isoparaffins are mixtures of non-polar organic solvents, not a single molecule. The makeup of this mixture can vary depending on the supplier and on the specific batch. They are branched (as indicated by the term “iso”) medium-chain alkanes. The paraffinic cleaning agents, typically containing chains of 9-15 carbons, may be referred to as hydrocarbon fluids or petrochemicals. We will discuss these cleaning products in more detail in future editions of Clean Source. The products are produced by distillation and further processing of crude oil or sometimes of natural gas. Short-chain alkanes are gaseous at room temperature, medium-chain alkanes are liquid, and longer-chain alkanes are waxy solids. The term “paraffin” is still commonly applied to waxy solids.
Alkenes and Olefins
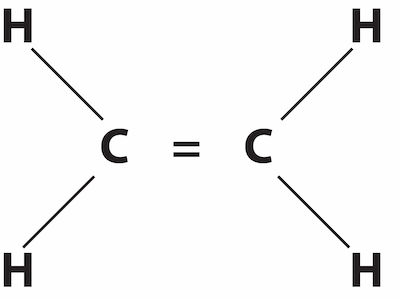 Ethene (ethylene)Alkenes and olefins are similar to alkanes in that they consist only of carbon and hydrogen, but with an important difference. Carbon can form bonds with up to four other atoms if all of the bonds are what are referred to as single bonds. Carbon can also form a double or even a triple bond. The simplest alkene, ethene, also commonly called ethylene, has two carbons and four hydrogens. In contrast, ethane, a saturated hydrocarbon, has three hydrogens attached to each carbon, and the two carbons are connected by a single bond. An alkene has only one carbon double bond, and an olefin may have multiple double-bonded carbons. Hydrocarbons with double or triple bonds are also referred to as unsaturated. The double and triple bonds are more reactive – less stable.
Ethene (ethylene)Alkenes and olefins are similar to alkanes in that they consist only of carbon and hydrogen, but with an important difference. Carbon can form bonds with up to four other atoms if all of the bonds are what are referred to as single bonds. Carbon can also form a double or even a triple bond. The simplest alkene, ethene, also commonly called ethylene, has two carbons and four hydrogens. In contrast, ethane, a saturated hydrocarbon, has three hydrogens attached to each carbon, and the two carbons are connected by a single bond. An alkene has only one carbon double bond, and an olefin may have multiple double-bonded carbons. Hydrocarbons with double or triple bonds are also referred to as unsaturated. The double and triple bonds are more reactive – less stable.
Chlorinated, Brominated, and Fluorinated Hydrocarbons
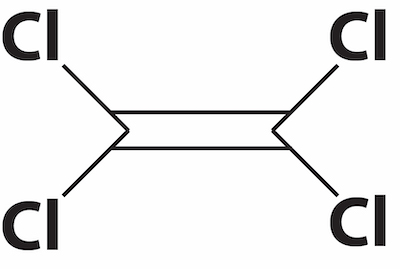 Perchloroethylene TetrachloroethyleneHalogenated hydrocarbons are a class of alkanes or alkenes where one or more of the hydrogens are replaced with a halogen (mostly Fluorine, Chlorine, and Bromine). An example of halogenated hydrocarbons that is used for cleaning is perchloroethylene, frequently referred to as “perc” or PCE. The prefix per means “all,” so perc is like ethylene, but where all four hydrogens have been replaced by chlorine. Because the double bond in PCE is more reactive than a single bond would be, PCE can break down to form trichloroethylene (TCE). TCE is sometimes referred to as a “daughter” compound of PCE.
Perchloroethylene TetrachloroethyleneHalogenated hydrocarbons are a class of alkanes or alkenes where one or more of the hydrogens are replaced with a halogen (mostly Fluorine, Chlorine, and Bromine). An example of halogenated hydrocarbons that is used for cleaning is perchloroethylene, frequently referred to as “perc” or PCE. The prefix per means “all,” so perc is like ethylene, but where all four hydrogens have been replaced by chlorine. Because the double bond in PCE is more reactive than a single bond would be, PCE can break down to form trichloroethylene (TCE). TCE is sometimes referred to as a “daughter” compound of PCE.
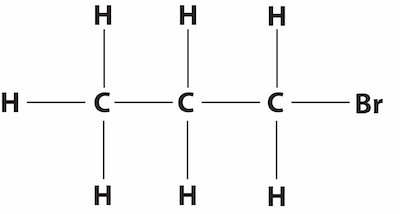 n-propyl bromide, 1-bromopropaneAnother example is n-propyl bromide (nPB), which is a 3-carbon propyl group with a Bromine atom replacing a hydrogen atom at the end. The IUPAC name for nPB is 1-bromopropane (1-BP) because the bromine is at carbon #1 of the three and is the name used by the EPA.
n-propyl bromide, 1-bromopropaneAnother example is n-propyl bromide (nPB), which is a 3-carbon propyl group with a Bromine atom replacing a hydrogen atom at the end. The IUPAC name for nPB is 1-bromopropane (1-BP) because the bromine is at carbon #1 of the three and is the name used by the EPA.
Alcohols
Alcohols have an OH group attached to an alkane. The naming system for simple alcohols is analogous to that for hydrocarbons. The shorter the hydrocarbon portion, the more polar the alcohol and the more miscible it is with water. Alcohols with longer hydrocarbon chains are more effective in dissolving oils and are less miscible in water.
If the OH group is attached at the end of the alkane, it is called normal alcohol; if it is attached somewhere in the middle, it is an iso alcohol. So, isopropyl alcohol (IPA) has the OH group attached to the middle of the three carbon atoms.
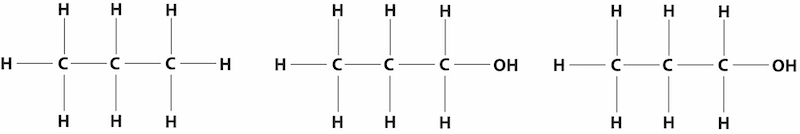 Propane (3 carbons); 1-propanol (normal-propyl alcohol); and 2-propanol (iso-propyl alcohol).
Propane (3 carbons); 1-propanol (normal-propyl alcohol); and 2-propanol (iso-propyl alcohol).
The number of carbons, hydrogens, and oxygens for 1-propanol and 2-propanol are the same. As Dr. Darren Williams explains using his football/basketball comparison, the arrangement of atoms in the molecule determines the shape; and the shape impacts the function. For example, 1-propanol has a boiling point of 97°C; 2-propanol boils at 82.5°C.
Modified Alcohol
Modified alcohols, or modified alcohol-based solvents, are more complex than IPA. One modified alcohol being used as a cleaning solvent has the formula CH3CH2CH2CH2OCH2CH(OH)CH3. As you can imagine, the structure is more complex than, say, IPA. Because the modified alcohol is much larger than IPA, the boiling point is considerably higher. While IPA boils at 82.5°C, modified alcohol boils at approximately 161°C. Based on the boiling point alone; we would expect IPA to be suited to benchtop/hand wipe cleaning while the modified alcohol would not.
We will discuss cleaning agents containing modified alcohol in more detail in a subsequent article.
Water
 WaterWater, H2O, is not an organic chemical because it does not contain carbon. By the way, the IUPAC name for water is oxidane (sounds more like a chemical, doesn’t it!). Note the naming convention similarity to alkanes.
WaterWater, H2O, is not an organic chemical because it does not contain carbon. By the way, the IUPAC name for water is oxidane (sounds more like a chemical, doesn’t it!). Note the naming convention similarity to alkanes.
Because the water molecule is not symmetric, water is polar, having a negative charge on the oxygen side and a positive charge on the hydrogen side. It has many different physical and chemical characteristics than organic alkanes and alkenes. We often write about aqueous cleaning, and we will continue to do so.
For a deeper dive into aqueous cleaning, our Aqueous Cleaning On-Demand course is now available from the Product Quality Cleaning Workshop (PQCW). For more information and to enroll https://shsu.geniussis.com/Registration.aspx?AffiliateID=J85GN6
Barbara and Ed Kanegsberg founded BFK Solutions in 1994 as a critical cleaning consulting service and the go-to resource to make cleaning, surface quality, and contamination problems go away or — even better — to avoid problems in the first place. Barbara, widely known as “The Cleaning Lady,” is an expert and trusted adviser in critical cleaning. Ed is known as “The Rocket Scientist,” and they write Clean Source, an approximately monthly e-newsletter that provides practical ideas to improve cleaning, contamination control, and product quality. They are co-editors of and contributors to the acclaimed two-volume “Handbook for Critical Cleaning,” CRC/Taylor & Francis, 2011. Visit https://bfksolutions.com

































