The term “degreaser” is confusing as any cleaning chemistry or cleaning process that removes oils and greases is doing the task of degreasing.
 Barbara and Ed KanegsbergDegreasing chemicals might be water-based with additives promoting non-polar substance dissolution. They may be organic solvents that inherently have solubility for oils. The term degreaser is also used for the cleaning equipment. Degreasing equipment might be as simple as a bucket containing a degreasing chemical; some consider a bucket of aqueous cleaning agents to be a degreaser.
Barbara and Ed KanegsbergDegreasing chemicals might be water-based with additives promoting non-polar substance dissolution. They may be organic solvents that inherently have solubility for oils. The term degreaser is also used for the cleaning equipment. Degreasing equipment might be as simple as a bucket containing a degreasing chemical; some consider a bucket of aqueous cleaning agents to be a degreaser.
However, this article narrows the discussion to a class of equipment termed “vapor degreasers.”
This equipment is used with solvents (organic solvents). You will learn about more traditional “open-top” degreasers and sealed, reduced-pressure systems (sometimes called “airless” degreasers) that show promise in intense regulatory situations. Notably, under recent US EPA amended TSCA activities and proposed rules, worker exposure limits are far lower than we are accustomed to (1).
(Story image above provided by Jayco Cleaning Technologies)
What is an Open-Top Vapor Degreaser?
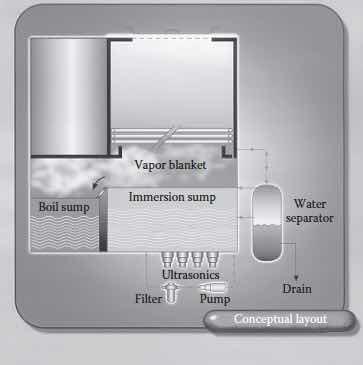 Diagram of an open-top vapor degreaser (From “Handbook for Critical Cleaning,” B. Kanegsberg and E. Kanegsberg, editors, CRC Press,2011)An open-top vapor degreaser includes an immersion chamber, a boil chamber (or sump), and a series of cooling coils (See Figure 1). It is referred to as a vapor degreaser because the “cloud” of solvent vapor that forms above the tank of boiling solvent can be used to clean, or more often rinse, a part suspended in the vapor zone. If a suspended part is cooler than the boiling point, vapor condenses on the part, just as steam condenses on a cold window. The liquid condensate then drips back into the solvent sump, carrying any dissolved oils, thereby cleaning the part.
Diagram of an open-top vapor degreaser (From “Handbook for Critical Cleaning,” B. Kanegsberg and E. Kanegsberg, editors, CRC Press,2011)An open-top vapor degreaser includes an immersion chamber, a boil chamber (or sump), and a series of cooling coils (See Figure 1). It is referred to as a vapor degreaser because the “cloud” of solvent vapor that forms above the tank of boiling solvent can be used to clean, or more often rinse, a part suspended in the vapor zone. If a suspended part is cooler than the boiling point, vapor condenses on the part, just as steam condenses on a cold window. The liquid condensate then drips back into the solvent sump, carrying any dissolved oils, thereby cleaning the part.
Many, if not most, vapor degreasers also use immersion cleaning and may include ultrasonics. A spray wand removes soils in difficult areas in many instances, particularly with complex parts and tight spacing. A key feature of vapor degreasers is that the condensate because it is freshly distilled from the boiling liquid, is uncontaminated; with liquid cleaning, only the first immersed part is cleaned in fresh solvent. Subsequently, the solvent contains material dissolved from earlier immersed parts. Some systems are manually operated; a worker controls the movement of the individual part. More sophisticated systems employ robotics to control the movement of parts within the system. This can be critical to minimize disturbance of the vapor blanket as parts are lowered or raised through the vapor zone.
Open-top degreasers should be optimized to the specific cleaning agent under consideration. Not all open-tops can be used for all solvents. It depends on the physical and chemical properties of the cleaning agent in question. For example, one newer cleaning agent, marketed as Solstice, is best used in a specially designed tightly sealed degreaser to avoid excessive solvent loss because it has a low boiling point (68°F). For degreasing in a flammable solvent like isopropyl alcohol, a vapor degreaser designed with appropriate engineering controls is imperative.
An open-top degreaser may be called a “closed-loop” because much of the cleaning chemistry is recaptured and condensed for repeated use. Some open-top degreasers have covers that close automatically, and others have tightly fitting lids. However, if there is ever an interface between the liquid or vapor and the surrounding air, these cleaning machines are termed “open-top vapor degreasers.”
Airless or Reduced Pressure Cleaning Systems
In contrast, sealed systems have been available for decades, with no interface between the air and solvent. They are sometimes termed “airless” and are operated at reduced pressure to ensure no emission into the surrounding air. When the process chamber is open to load or unload parts, there is no detectable liquid or vapor solvent interface with the air. These sealed, reduced pressure systems can also be used for cleaning operations that don’t necessarily include vapor condensing, such as for containing flammable or combustible fluids, including modified alcohols and iso-paraffins (2).
As with open-top systems, sealed, reduced-pressure systems are best optimized for the cleaning agent, and not all machines can be used with all solvents. In addition, these systems require a higher initial investment and ongoing maintenance than open-top systems. Still and all, many such systems have been operating successfully for decades.
Why Use an Airless or Reduced Pressure System?
Technical reasons include more consistent process control (including modulating the boiling point) and lower solvent usage. Instead of standing over a degreaser, you load the parts to be cleaned into an empty chamber, start the cleaning cycle, walk away (maybe take a coffee break?), open the machine – and see clean, dry parts – no solvent.
One primary driver has been minimizing solvent losses to the air, an especially important issue in areas of poor air quality; solvent losses can be near zero. In addition, because there is no vapor or liquid/air interface, employee exposure is minimal.
Particularly given the ECELs developed under EPA amended TSCA (1), understanding and documenting achieved employee exposure. This is an issue. U.S. EPA seems to want demonstrations of employee exposure levels (3). Manufacturers of these systems may know that levels below that current U.S., State, and industry can be achieved. However, equipment suppliers and those using the systems may be reluctant to do measurements.
For one thing, results may vary depending on the complexity of the product being cleaned. Highly complex parts might contain some solvent under some conditions. In addition, historically, regulatory agencies have demanded lower and lower levels of solvent release – it has become a never-ending (probably futile) game of “limbo.”
Could or should all open-top degreasers go away? While an eventual move to a reduced pressure system is often desirable, such systems may not always be practical or affordable, particularly for small shops. If amended TSCA proceeds according to proposed rules, using personal protective equipment (PPE) might be an expeditious way to achieve low worker exposure levels. Some companies have adopted this approach; it may not be the best way to consistently protect workers. PPE is cumbersome, requires training, and may not be used properly.
Why the World Needs a Definition For “Sealed, Reduced Pressure” Systems
We are developing a definition encompassing the design and purpose of these sealed systems. We have avoided using the term “airless” because that term can imply a total vacuum. Although the pressure is reduced, it may or may not need to reach a strong vacuum. We are attempting to put together such a definition because some people, including those in the regulatory sector, may not clearly distinguish between an open-top system with a tight lid and a reduced pressure system. Reduced pressure systems are often referred to as “closed-loop.” We’ve seen this in the literature of such sealed systems provided by manufacturers. All vapor degreasers are closed-loop and recycle the solvent.
Our Working Definition
Barbara defines a sealed, reduced pressure system as a “Roach Motel” for solvent – the solvent goes in but can’t get out. Realizing that more technical detail is desirable, we have developed a working definition for what is sometimes called airless systems, building on the definition used by the Southern California Air Quality Management District (SCAQMD) (4).
Given the general regulatory move toward lower and lower exposure limits and concern with the possible or perceived environmental and worker exposure concerns with all chemicals, understanding and defining this technology may be important to the future of product manufacturing.
“Sealed, reduced pressure cleaning systems” are a category of cleaning systems typically used for solvent or solvent blends. Some are available for water-based cleaned agents. When the process chamber is open to load or unload parts, there is no detectable liquid or vapor solvent interface with the air. The system is sealed and automated under reduced pressure. During the cleaning process and normal operation conditions, people are not in contact with the cleaning agent or product being cleaned.
The systems are designed to achieve near-zero discharge of volatile vapors to the atmosphere during cleaning, rinsing, and drying, as well as between runs. The systems include devices to condense and recover cleaning agents from the liquid and vapor phases. The systems include control devices to remove or capture solvent vapors from all streams that vent to the atmosphere.
(Story image above provided by Jayco Cleaning Technologies)
References
- B. Kanegsberg and E. Kanegsberg, “Degreasing and Fireworks,” Clean Source, The Official Journal of the Cleaning Lady and the Rocket Scientist, July 2022. https://bfksolutions.com/degreasing-and-fireworks/
- D. Williams, B. Kanegsberg and E. Kanegsberg, “Cleaning with Organic Solvents, Part 2: Iso-paraffins and Modified Alcohols,” Clean Source, The Official Journal of the Cleaning Lady and the Rocket Scientist, April, 2023. https://bfksolutions.com/cleaning-with-organic-solvents-part-2-iso-paraffins-and-modified-alcohols/
- Proposed EPA rule for Perchloroethylene https://www.regulations.gov/document/EPA-HQ-OPPT-2020-0720-0024
- SCAQMD Rule 1122, “Solvent Degreasers,” http://www.aqmd.gov/docs/default-source/rule-book/reg-xi/rule-1122-solvent-degreasers.pdf?sfvrsn=4
Barbara and Ed Kanegsberg founded BFK Solutions in 1994 as a critical cleaning consulting service and the go-to resource to make cleaning, surface quality, and contamination problems go away or — even better — to avoid problems in the first place. Barbara, widely known as “The Cleaning Lady,” is an expert and trusted adviser in critical cleaning. Ed is known as “The Rocket Scientist,” they write Clean Source, an approximately monthly e-newsletter that provides practical ideas to improve cleaning, contamination control, and product quality. They are co-editors and contributors to the acclaimed two-volume “Handbook for Critical Cleaning,” CRC/Taylor & Francis, 2011. Visit https://bfksolutions.com


































