Chromate conversion coating is a type of conversion coating applied to passivate aluminum in order to slow corrosion.
The process uses various toxic chromium compounds, which may include hexavalent chromium. Industry is developing less toxic alternatives in order to comply with substance restriction legislation such as RoHS. One alternative is trivalent chromium, which may not be as effective as hexavalent chromium, though it is less environmentally damaging. This paper will describe a new trivalent chromium process for chromate conversion on aluminum with outstanding results. It is QPL (Qualified Product List) approved from the United States Navy - Defense Standardization Program under Governing Spec MIL-DTL-81706B.
Introduction
This paper will outline various chromate conversion techniques for aluminum. It will address a new environmentally friendly, cost efficient and performance oriented chromate conversion coating with a unique and patented trivalent chromium pre and post treatment chemistry for aluminum.
Chromate Conversion of Aluminum
Chromate conversion coatings are applied to passivate aluminum, zinc, cadmium, copper, silver, magnesium, tin and their alloys to slow corrosion. To achieve their full protective properties on aluminum alloys, most organic coatings require the metal surface to be pre-treated. This provides a surface film, which becomes an integral part of the metal surface involving virtually no dimensional change. Chromate coatings, similar to phosphate coatings, are processes of chemical conversion. However, the chromate coatings are formed by the reaction of water solutions of chromic acid or chromium salts. The coatings can be applied to aluminum, zinc, cadmium, and magnesium. The coatings usually have good atmospheric corrosion resistance. These conversion coatings form an ideal substrate for paints and precious metals, providing a clean, essentially inert surface, which gives optimum adhesion. The application of chromated aluminum can cover a wide range of functions. Conversion coating can provide mild wear resistance, better drawing or forming characteristics and may be used to provide as a decorative finish and is also ideal for pre-treatment prior to organic coating.
Most organic coatings applied directly to aluminum surfaces will not adhere well, and if subjected to any deformation, they will tend to flake off exposing the bare aluminum. Scratching off the paint surface would also provide a nucleation site for aluminum corrosion and further undercutting of the coating.
The successful application of this conversion process requires the aluminum to be clean and free from organic soils, oxides and corrosion products. Therefore a pre-treatment is required which can be applied to aluminum, and provides a suitable basis for subsequent coatings. Conversion coatings have been developed which can be used on aluminum alloys and are compatible with most paint systems. The name conversion coating describes a process of chemical reaction that results in a surface film. As a result of this reaction and conversion the film becomes an integral part of the metal surface, which exhibits excellent adhesion properties. Chromate conversion coatings are a thin chemical film, usually less than 0.25 microns in thickness. They are electrically conductive.
Hexavalent Chromates
Historically, hexavalent chemistry has been used to process aluminum chromate conversion parts. There are some benefits with hexavalent chemistry. Chromate passivation systems containing hexavalent chromium compounds are an extremely versatile group of aqueous chemistries that are extensively used in a diverse range of electroplating and metal treatment processes. They impart many beneficial and essential characteristics to metallic substrates and to deposits obtained from a number of techniques such as zinc electroplating. Hexavalent- based passivation (Cr+6) exhibits a number of desirable characteristics. They will passivate the surface of zinc and zinc alloy electrodeposits with a thin film that provides end-user benefits such as color, abrasion resistance and increased corrosion protection. When damaged, these hexavalent chromates possess a unique "self healing" property. This means that soluble hexavalent chromium compounds contained within the passivation films will re-passivate any exposed areas. Hexavalent chromate has wet, gelatinous film, drying at the surface. Subsurface moisture (dehydrating in approximately 48 to 72 hours) provides self-healing and lubricity characteristics. The deposits are harder than conventional trivalent chromate film. It offers torque and tension to meet fastener’s finishing requirement. Hexavalent chromium products are carcinogenic substances. They are hazardous to human health and environment. Hexavalent chromates leach into the grounds and cause damage. It is prohibited due to its toxic nature. Environmental guidelines and regulations have been in place to restrict and prohibit its usage.
Desirable Characteristics of Hexavalent Chromate Passivates
- Prevents Oxide Formation
- Provides Color
- Slow corrosion in prototypic tests (e. g. salt spray, rooftop, etc.)
- Provides adhesion for organics (e. g. paint)
- Helps prevent corrosion of painted surface
- Conductive
- Thin
- Flexible
- Lubricious
- Easily applied
- Stable for weeks or months
- Durable
- Resilient (self healing)
- Coats in recesses
- Easy to strip
- Inexpensive equipment
- Single tank
- Inexpensive (charge up cost)
The industry is developing less toxic alternatives in order to comply with substance restriction legislation and directives from the European Union. The most affecting directive is Restriction of Hazardous Substance (RoHS), 2002/95/EC of the European Parliament and The Council of the European Union, signed on 27 January 2003, which went into effect July 1, 2006. It covers six hazardous substances - lead, mercury, cadmium, hexavalent chromium (Cr+6), PBB (polybrominated biphenyls) and PBDE (polybrominated diphenyl ether). Another European Union directive, the second directive that also contains hexavalent chromium, is End of Life Vehicle (ELV) directive, 2000/53/EC of the European Union & the Council of European Union, was signed on September 18, 2000 and went into effect on July 1, 2007. Four heavy metals included in ELV directive are - cadmium, lead, mercury and hexavalent chrome (Cr+6), (approximately 70% of total heavy metals is Cr+6).
Industry has been actively following any new development to replace hexavalent chromium. The most common alternative is trivalent chromium, which is environmentally friendly. However, there are still some weaknesses with trivalent chromate coating. In order to achieve equal or better corrosion resistance as compared to hexavalent chromate, in most cases a sealer or a topcoat is required. Some chemical manufacturers now offer better salt spray performance without any sealers or topcoats. Trivalent chromates are not self-healing. Their bath life is shorter than hexavalent chromate bath, requires 1400 F. operating temperature, and do not offer identical colors. In recent years there have been new developments in trivalent chemistries. More colors are now available and coating’s performance has significantly improved specially for corrosion resistance. Typical trivalent chromate film has a pale greenish color. Trivalent chromate deposits are electrically non-conductive (unless applied over a zinc alloy or a metallic substrate).
The most significant development for the replacement of hexavalent chromates is the trivalent chromium pretreatment (or post treatment), developed by the United States Navy, Naval Air Systems Command (NAVAIR). This is a unique chemistry, specially formulated and developed for aluminum. NAVAIR has spent over two and a half years and tested more than 15,000 panels for development of the product. This formulation contains less than one percent of trivalent chromium and operates at ambient temperatures (650 F. – 850 F.). It does not contain any restricted or hazardous substances and as a result, does not require any exhaust systems. Most importantly, it complies with ALL European Union directives including Restriction of Hazardous Substance (RoHS), End of Life Vehicle (ELV), and Waste Electric and Electronic Equipment (WEEE). It has shown outstanding performance as compared to other conventional trivalent chromates. This trivalent chromate chem. film is harder than conventional trivalent chromate. It is electrically conductive (LER – low electrical resistance) and it meets or exceeds ASTM D2559-9, MIL-DTL-5541 F and MIL-DTL-81706 for electrical resistance. Therefore it is useful in electric and electronic equipment where surface resistivity is critical and required. It has excellent adhesion and bonding properties and provides an undercoat for organic coatings such as paints. It meets or exceeds dry tape adhesion requirements for ASTM D 3359 method A & B. This trivalent chromium pretreatment is an ideal undercoat for cured coatings and overcoat for plated materials that require subsequent hydrogen relief.
This unique trivalent chromium pretreatment can be exposed to temperatures exceeding 8000 F following a 24 hours cure period. It can be baked for hydrogen relief in excess of 5000 F for more than 24 hours without loss of performance while hexavalent chromates cannot be baked above 1400 F without loss of performance. In most cases, its corrosion resistance performance is equal to or better than conventional trivalent chromates and also hexavalent chromates.
Results of 168 to 500 hours can be achieved in neutral salt spray testing (ASTM B117), depending upon the aluminum alloy tested. In short, this trivalent chemistry from NAVAIR offers an overall superior performance without any sealer or a topcoat.
NAVAIR’s trivalent chromium treatment has also been used to replace high and mid temperature anodize seals. It has proven to be environmentally friendly and efficient anodize seal without any hazardous chemicals. Trivalent chromium pretreatment (from NAVAIR) is QPL (Qualified Product List) approved from the United States Navy - Defense Standardization Program under Governing Spec MIL-DTL-81706B (1) "Chemical Conversion Materials for Coating Aluminum and Aluminum Alloys".
Body
The United States Navy, NAVAIR, has formulated and developed their patented and unique trivalent chromium pre and post treatment chemistry. Their results were very encouraging. Most aluminum alloys were tested and able to achieve up to 500 hours of neutral salt spray (NSS) with concentration of trivalent chromium pretreatment material ranging from 15 to 25 % by volume with various time cycles that ranged from 3 to 5 minutes at ambient temperatures. This new chemistry has been pursued due to its tremendous success in initial testing. This product has been developed for aluminum and all alloys were tested. It is a drop- in replacement for hexavalent chromate. As mentioned earlier, due to the low concentration and chemistry of non-hazardous substances, it can be operated in manufacturing environment without any requirement for exhaust system.
The pretreatment of aluminum is important to achieve desired specification. Alloys such as 6061 and 7075 are relatively easy to process and can work with most available detergent and activation agents, acids or deoxidizers. On the other hand, 2024 alloy (with up to 5% copper content) is sensitive and requires specific pretreatment. The nature of this alloy makes it sensitive to some etching cleaners and may require certain acids to activate the part surface. This patented trivalent chemistry by NAVAIR, is simple to operate provided that you pay attention to the pretreatment and also to pH of the bath. The normal operating pH range of this bath is 3.6 to 4.0. A pH that is lower than the operating range may cause early corrosion in salt spray testing as the bath becomes more aggressive. A combination of lower pH and slightly elevated temperature really makes it aggressive and supports a shorter time cycle. Studies were done by a licensee of NAVAIR’s trivalent chromium conversion coating which examined test results of various aluminum alloys treated with different process cycles. It included various time, temperature and concentration of more than one detergent. Special attention was given to the etching nature of some of the detergents. The objective was to achieve a clean surface without any modification or powdery film. After cleaning, the surface was activated by various chemical methods of which were chosen based on the alloy. Also, important factors included concentration and cycle time in activation bath. Rinsing was also given special attention. Tap water or de-ionized water was selected as required. Quality of rinse water such as the levels of chlorides and total dissolved solids were observed. Trivalent chromium pretreatment bath was operated at ambient temperatures as well as at elevated temperatures for evaluation purposes. Testing of different alloys included different concentration of trivalent chromate bath. It also included different time cycle.
The results were based on the performance in neutral salt spray chamber. We evaluated different test matrices and found that certain alloys required a specific overall treatment. Alloys, which have been not so difficult to process, offered us excellent results. Salt spray hours in neutral salt spray ranged from 168 to 800 hours. When a specific process cycle was followed, 2024 alloy also performed to salt spray hours ranging from 168 to more than 1000 hours.
Enhanced Performance Additive
One of the most daunting aspects of trivalent conversion coatings as a drop in replacement for hexavalent chromium chemistries is the requirement of rigid pretreatment parameters in order to see maximum corrosion performance. Across the board, job shops and formulators alike go to great lengths to produce consistent results that pass requirements such as MIL-DTL-5541 and other corrosion specifications. Application facilities not well equipped to perform experiments for pretreatment optimization are finding themselves making small beaker size batches of chemistry with the intention of scaling up to their production line. This is good practice when tight controls are met with proper standards but can be convoluted for some and more work than what a typical job shop is used to when integrating a new product into their line. The standard practice in industry is to purchase a material, follow the operating parameters and voilá it works. Trivalent chromium conversion coatings are not, in general, that straightforward. The nature of the technology poses the burden of tailoring the facility’s resources and equipment constraints to the product, which requires a slightly more sophisticated level of understanding. This may come as a shock to those transitioning to trivalent chromium chemistry and can often be discouraging. There have been successful attempts in creating a more robust process to allow leeway in pretreatment parameters. Research institutions and private industry groups are looking for additives to put in trivalent chromium conversion coating baths. Anyone with experience using trivalent conversion coatings knows that the more surface modification done to a substrate, such as etching, can cause a drastic decrease in corrosion resistance. A study of one particular additive indicates an improvement rate for corrosion resistance of 50% on etched substrates and 38% on non-etched substrates when using an enhanced bath (See Figure 1). The study included 163 different processes that utilized several different cleaners, deoxidizers, and etchants at various temperatures, concentrations and cycle times. All panels treated in the enhanced protection additive (EPA) were tested against a standard, un-enhanced (NAVAIR) trivalent chromium pretreatment bath. Test panels were sent to an outside party, NADCAP certified laboratory for neutral salt spray test (ASTM B117) as well as examined in-house. Panels were examined at intervals of 96, 168 hours, 212 hours, 267 hours, 336 hours and 407 hours and beyond. For the sake of brevity, only one parameter will be reported in this document: etched Vs non-etched. The total numbers of test panels were in the hundreds.
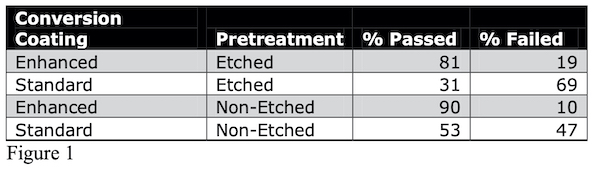
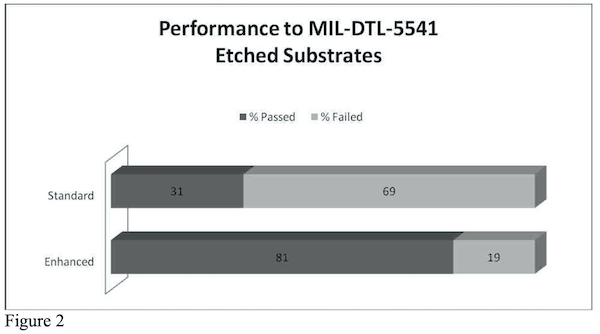
The data in Figure 2 indicates that, statistically, out of 100 different process variations, 81 of them will pass MIL-DTL-5541 on etched substrates using an enhanced version of a trivalent conversion coating while only 31 process variations will pass using a standard, un-enhanced trivalent chromium chemistry.
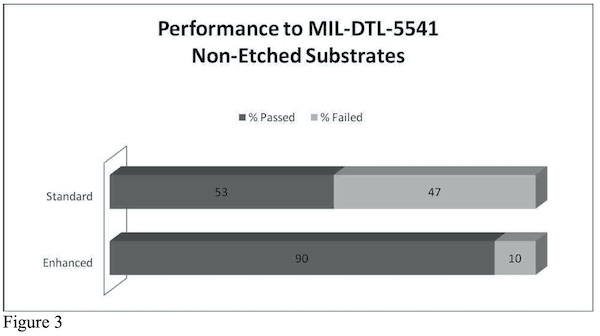
Though the improvement on non-etched substrates is not quite as dramatic as it is for severely modified substrates, using an additive in a tri-chrome bath is still beneficial. The data in Figure 3 indicates that statistically, out of 100 different process variations, 90 of them will pass using an enhanced version and only 53 will pass using a standard, un-enhanced bath.
Discussion
Extended protection additive (EPA) performs much better than standard trivalent chromate bath (NAVAIR). The addition of EPA in a standard trivalent chromate pretreatment bath varied from 15% to 30%. We had used addition of 25% EPA in a standard trivalent chromate bath for our test evaluation. The objective here is to study the influence of extended protection additive in a standard (NAVAIR) trivalent chromate pretreatment bath and to evaluate the difference in performance with the addition of extended protection additive. In order to evaluate this, we had to set up a test plan and select appropriate chemistry. We had selected the 2024 aluminum alloy as our baseline standard. As you probably know, 2024 contains up to 5% of copper, which at times could be an issue for corrosion resistance. We had assumed that if we can achieve superior results on this alloy, the other alloys such as 5052, 6061 and 7075 would perform as well or better than 2024 alloy panels. We wanted to study that the extended protection additive helps enhance corrosion resistance performance of trivalent chromium pretreatment on aluminum.
The detergents for this trial were selected based on some major customers’ suggestions as well as our past history of various testing with trivalent chromium pretreatment applications. The temperature was one of the important factors that were controlled in a range of 1200 to 1250 F. As much as cleaning of a part surface is important, it is also important to clean the surface without drying out during the transfer stage. The concentration of these detergents was based on chemical manufacturers’ suggested operating range at the lower end. The results of neutral salt spray (NSS) did not indicate any reasons for any of the detergents responsible for any failure. It is therefore concluded that the process parameters for all different detergents were acceptable as tested for test evaluation with the extended protection additive in a standard trivalent chromium pretreatment bath. The test panels were etched using a selected etchant as suggested by major customers. The study was involved around the difference in performance with and without the etchant for extended protection additive performance. The results were favorable for the use of this etchant in a trivalent chromium pretreatment bath with EPA additive. The process parameters were kept at the same level for etched and non-etched test panels for comparison. Out of the 164 total test panels, 119, or 73% of the etched panels passed. There were fewer failures for the etched panels (22%) with the EPA than without it in a standard trivalent chromate pretreatment bath. On the other hand, there were fewer failures of non-etched panels (8%) when used with the extended protection additive than when used in a standard trivalent chromium pretreatment bath. It was concluded from this study that the extended protection additive (EPA) increases corrosion resistance by 51% on etched panels. It was also concluded that EPA increases corrosion resistance by 39% on non-etched panels. This data shows that this extended protection additive helps to enhance corrosion resistance performance with or without the etchant as compared to a standard (NAVAIR) trivalent chromium pretreatment application.
The results from neutral salt spray test performed by a NADCAP certified laboratories showed that standard trivalent chromium pretreatment (NAVAIR) performed from 96 hours to 768 hours with the etched panels. The non-etched panels in the same bath performed better in neutral salt spray (NSS) with the hours ranging from 168 to 2786 hours. The same process for evaluation of panels tested for the extended protection additive (EPA) showed that etched panels performed in neutral salt spray (NSS) from 174 hours to 912 hours, while non-etched panels performed from 72 hours to more than 3120 hours. To activate the part surface, an acid in a combination with a deoxidizer was used. There were different mix ratios of acid and deoxidizer as well as the process cycle time for test purpose. Results were based on various combinations of cycle time and concentration ratios.
Neutral salt spray results indicated that lower concentration of acid in a deoxidizer performed better than higher concentration of acid/deoxidizer for the standard trivalent chromium pretreatment (NAVAIR) bath. However, acid concentration did not substantially influence the trivalent chromium pretreatment with EPA additive treated substrates. Test panels processed with low concentration of acid/deoxidizer in trivalent chromium pretreatment (NAVAIR) bath failed at 168 hours, while test panels with EPA additive passed 267 hours.
Summary
Trivalent chromium pretreatment formulated and invented by NAVAIR performs very well for replacing hexavalent chromium on aluminum. Its performance varies depending on the type of detergent, its concentration, temperature and cycle time. Also important is the surface activation and type of the acid and/or deoxidizer that is used for the application. However, the most important is the type of aluminum alloy for chromate conversion coating. It was concluded from the study that 2024 is the most difficult alloy due to its metallurgical content and if satisfactory results can be obtained for 2024, the other aluminum alloys are not so difficult to achieve successful results.
The extended protection additive, when added in the standard trivalent chromium pretreatment (NAVAIR) bath, has shown outstanding performance in neutral salt spray. This additive is designed to enhance corrosion resistance of aluminum alloy for trivalent chromium pretreatment performance. EPA offers consistent performance for difficult alloy such as 2024 for corrosion resistance. It is an additive that improves standard NAVAIR trivalent chromium application on aluminum for robust performance.
It is sufficient to say that the most important aspect of using this newly developed, patented additive for trivalent chromium pretreatment is the robustness for consistent performance for corrosion resistance. It is QPL (Qualified Product List) approved from the United States Navy - Defense Standardization Program under Governing Spec MIL-DTL-81706B (1) "Chemical Conversion Materials for Coating Aluminum and Aluminum Alloys".
Visit chemeon.com for additional information
References
1. Wernick, S., Pinner R. and Sheasby P. G. Fifth Edition, Volume 1, 1996. The Surface Treatment and Finishing of Aluminum and its Alloys
2. Wernick s., Pinner R. and Sheasby P. G. Fifth Edition, Volume 2, 1996. The Surface Treatment and Finishing of Aluminum and its Alloys
3. Formation of chromate conversion coatings on aluminum and its alloys: K. Sasakia, H.S. Isaacsb, C.S. Jaffcoatea, R. Buchhaita, V. Legata, H. Leeb, V. Srinivasamurthic, Bralla, J.G. (ed.) (1986), Handbook of Product Design for Manufacturing, McGraw-Hill (New York).
4. Edwards, Joseph (1997). Coating and Surface Treatment Systems for Metals. Finishing Publications Ltd. and ASM International, pp. 66-71
5. Bishop, et al. Galvanotechnik, 71(1980) Nr. 11, p. 1199.
6. European Union End-of-Life Vehicle Directive 23 May 2000 8828/00 (Presse 179): Further details can be obtained from the Council site at http://ue.eu.int/Newsroom.
7. Wood G. C. and O’Sullivan J. P., Electrochem Society, 1969, 47,142- 144
8. Sheasby P. G. and Bancroft G., Trans. Inst. Met. Finishing, 1970, 48, 140-144