Automotive conversion coatings consist of layers of materials that are chemically applied to the body structures of vehicles before painting to improve corrosion protection and paint adhesion.
These coatings are a consequence of surface-based chemical reactions and are sandwiched between paint layers and the base metal; the chemical reactions involved distinctly classify conversion coatings from other coating technologies. Although the tri-cationic conversion coating bath chemistry that was developed around the end of the 20th century remains persistent, environmental, health, and cost issues favor a new generation of greener methods and materials such as zirconium. Environmental forces driving lightweight material selection during automobile body design are possibly more influential for transitioning to zirconium than the concerns regarding the body coating process. The chemistry involved in some conversion coatings processing has been known for over 100 years. However, recent advances in chemical processing, changes in the components used for vehicle body structures, environmental considerations and costs have prompted the automobile industry to embrace new conversion coatings technologies. These are discussed herein along with a historical perspective that has led to the use of current conversion coatings technologies. In addition, future directions for automobile body conversion coatings are discussed that may affect conversion coatings in the age of multi-material body structures.
1. Introduction
In a 1911 US Patent [1], Thomas Coslett described the use of six ounces of zinc, a pint of water, and a pint of phosphoric acid as ingredients for making a conversion coating concentrate. Its efficacy was demonstrated when iron objects boiled in a water solution made from this concentrate displayed a significant reduction in corrosion as a consequence of a protective zinc phosphate coating that had been formed on the surface of the iron.
The definition of a conversion coating is a coating formed during surface-based chemical reactions that include the base metal and other ions present in solution. Being a direct consequence of surface reactions differentiates a conversion coating from other coatings such as paint: paint is really a covering. Since 1911, vastly improved conversion coating compositions have included various elements in the periodic table and now include numerous organic and inorganic compounds.
Although the evolution of conversion coatings started with iron requiring more corrosion protection, other metals such as zinc and aluminum are also now beneficiaries of conversion coating technologies. For example, automotive bodies have incorporated Zn as a sacrificial anode in the form of galvanneal (GA), or electrogalvanneal (EG)-treated steel and Al, a lightweight substitute for steel. Paints, or other organic coatings, have improved adhesion characteristics because of a roughened surface that is created by the conversion coatings; in other words, a conversion coating also functions to improve the mechanical anchor profile for paint.
The canned food and beverage industries have exerted significant influence on the development of new metal ion chemistries for conversion coatings that have improved coating appearance after container pasteurization and the adhesion of decorative organic coatings. By the 21st century, environmental concerns weighed in on chromates, heavy metal sludge, phosphates, and energy consumption. The long-term combination of these forces have nudged conversion coating technologies to Zr-based materials, a trend noted by David Chalk of Dubois Chemical as the “shrinking periodic table” [2].
2. The Automotive Coating Stack
Figure 1 illustrates a typical stack of coatings that are applied onto an automotive body during the painting process [3]; the layers of these coatings add beneficial attributes as noted in the left-hand side of the Figure. However, a customer in an automobile dealer showroom only senses the upper three layers of the “paint job”, which include a clear coat, base coat or color (possibly with optional metal flakes) and a primer; these coverings are typically organic in nature. Layer 4 below the coverings is an electro-deposition (ED) coating, and layer 5 is the phosphate conversion coating. The activation layer below the phosphate layer—i.e., between the phosphate layer and the steel body panel—typically consists of Ti or Zn but does not have a thickness per se because it is made of noncontiguous particles applied under specifications that call for an application based on a weight-per-unit area.
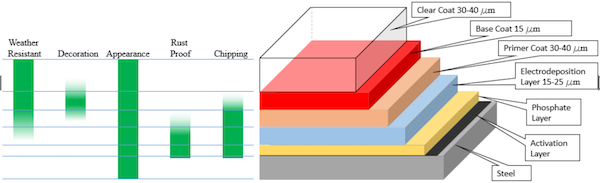
Figure 1. Vertical stack on an automotive body panel with a phosphate conversion coating (not drawn Figure 1. Vertical stack on an automotive body panel with a phosphate conversion coating (not drawn
to scale) [3]. to scale)
3. The Automotive Conversion Coating Timeline and Purpose
A timeline for phosphate conversion coating technologies is given in Figure 2; starting at around 1940, it notes some significant milestones [4]. The conversion coatings serve two primary purposes on vehicle bodies. First, they increase corrosion protection when compared to an untreated metal surface; second, they promote adhesion between the paint and metal, creating a more robust bond than in the case of paint applied directly to metal. Together, these effects act as a system that is more tolerant of chemical and mechanical attacks on the body panel surface.
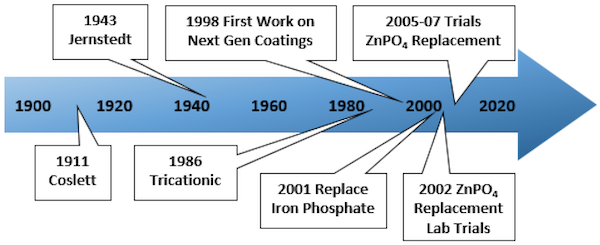
Figure 2. Significant milestones in the progress of phosphate conversion coating technology.
4. The Foundation of Zinc Phosphate Conversion Coatings
Figure 3 is presented because discussions found later in this article require some familiarity with the chemical reactions associated with the application of a conversion coating; these reactions include pH-driven precipitation, acid disassociation, oxidation, reduction and redox, the pH gradient boundary layer, and chromate rinsing. In its simplest form, a zinc phosphate coating starts with oxidation at a surface micro-anode when an Fe atom dissolves into the phosphoric acid solution as a Fe2+ cation and leaves behind two electrons on the base metal. Simultaneously, reduction occurs at a surface micro-cathode. The two electrons residing on the metal then combine with H+ in the solution to form surface-adsorbed hydrogen. These reactions are described in Equations (1) and (2) [5].
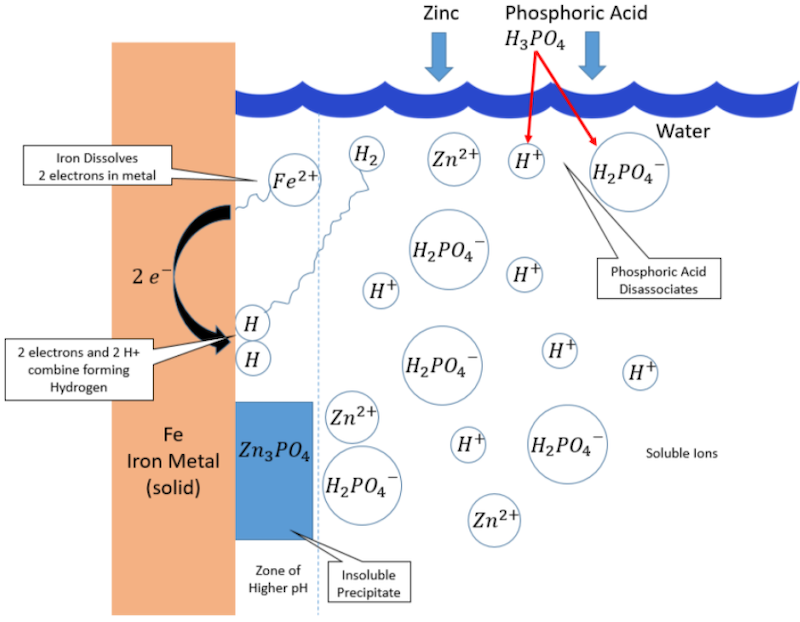
Figure 3. Simplified mechanism of zinc phosphate conversion coating (pH around 3).
To read the rest of this research, please CLICK HERE
Written by Mark Doerre 1,* Larry Hibbitts 2, Gabriela Patrick 2 and Nelson K. Akafuah 1
- 1 IR4TD, College of Engineering, University of Kentucky, Lexington, KY 40506, USA
- 2 Paint Production Engineering, Toyota Motor North America, Inc. (TMNA), Georgetown, KY 40324, USA
Appeared in Coatings 2018, 8(11), 405; https://doi.org/10.3390/coatings8110405



































