Corrosion is one of the biggest enemies of manufactured metal products, particularly in a demanding environment, e.g., traffic or agriculture.
As an example, water and dirt can enter the steering knuckle and initiate corrosion, which can cause the control arm to separate from the vehicle, increasing the risk of an accident (Liu et al., 2018). Although, the automotive industry is one of the most progressive industries in the world and its progress is beneficiary for other industries, areas can still be found to improve. One of these areas is surface treatment technologies.
 Ján LilkoVarious surface treatments, such as top coat application, primer application, ecoat treatment, zinc phosphating, and wax application, can protect the car from mechanical damage, weather conditions, and determine the final visual properties. They contribute to corrosion resistance as well. EN ISO 12944:2017 defines corrosion as a set of all changes in metallic materials caused by the effects of oxygen, water or electrolyte.
Ján LilkoVarious surface treatments, such as top coat application, primer application, ecoat treatment, zinc phosphating, and wax application, can protect the car from mechanical damage, weather conditions, and determine the final visual properties. They contribute to corrosion resistance as well. EN ISO 12944:2017 defines corrosion as a set of all changes in metallic materials caused by the effects of oxygen, water or electrolyte.
Despite all the efforts, experts estimate that corrosion will lose 3–5% of the national gross domestic product of developed countries each year (Goldschmidt and Streitberger, 2007). Therefore, corrosion can lead to tremendous financial losses. One of the most dangerous types of corrosion is galvanic or bimetallic corrosion. Galvanic corrosion occurs when a metal is electrically bonded to another, more noble metal, or conductive material is in contact with an electrolyte. Less noble metal corrodes.
Although galvanic corrosion can only occur when there is contact between two metals and an electrolyte is present, the components may not be in direct contact (Britner et al., 2021). During the joining of different types of materials, such as welding aluminium and steel, these two different metals create ideal conditions for bimetallic corrosion at the point of direct contact (Gullino et al., 2019). The reason for using different materials in the vehicle body is to achieve lower weight with increased toughness of the car (Fentahun and Savas, 2018). In the case of bimetallic joints of aluminium and steel in the automotive industry, it is also necessary to assess the strength of joints in a corrosive environment, because aluminium alloys generally have a higher electrode potential than steels (Reif, 2014). Aluminium electronegativity is -1.66 V, iron electronegativity -0.44 V, and zinc electronegativity -0.76 V.
The European Commission set up the critical target for CO2 emissions for 2030 to 75 g·km-1. Recently, the European Climate Law has been adopted, and the EU is committing to carbon neutrality by 2050. The low weight of the vehicle body has a direct effect on energy consumption and CO2 emissions. The key in reducing CO2 emissions is a multi- material body, which enables achieving an extremely low weight and reduced production costs (Cischino et al., 2017). This can be achieved by using new materials, new combinations of bimetallic joints, or by using suitable advanced methods of materials joining.
The joining of metallic materials with different electro potentials leads to an increased risk of galvanic corrosion. Surface treatments are technologies which increase investments and costs. It is therefore necessary to find cost-effective, but also suitable surface treatments that meet quality requirements. Salt spray corrosion testing is a common part of metal material verification. There are various forms of accelerated corrosion tests, including continuous salt spray tests specified by different standards, e.g., ASTM-B117-19 (American Society for Testing and Materials standard) and ISO 9227:2017.
This scientific article describes the preparation, implementation, and evaluation of the cyclic corrosion test of bimetallic joints of materials used in the construction of car bodies. The method and design of sample preparation is unique and has not yet been published in any literature.
Material and Methods
Preparation of testing samples
The testing plates widely used in the automotive industry by Chemetall were used to assess the quality of finishes. The dimensions of the testing plates were 105×190×1 mm. Ten pieces of Gardobond AA6014/two aluminium test plates and ten test plates made of galvanized steel brand Gardobond GB MBZ 1 were used.
A tucker ERF 23.2 riveting station has been used to rivet 10 sample pairs of different materials. Each sample was riveted and sealed with glue with a minimum overlap of 50 mm and 6 rivets per piece of sample. Figure 1 shows the aluminium and galvanized steel samples after the riveting process.
 Fig. 1 Aluminium and galvanized steel testing plates. Surface treatment of the testing samples – pre-treatment
Fig. 1 Aluminium and galvanized steel testing plates. Surface treatment of the testing samples – pre-treatment
The testing pair of plates has been taken out of the testing samples prior pre-treatment and ecoat.
The testing samples have been degreased in the pre- treatment bath at a temperature of 50°C–60°C and rinsed. Galvanizing in a zinc–phosphate solution at 45°C for 2.5 minutes has been performed. The testing samples have been rinsed and passivated in a Henkel Bonderite 54 NCA bath at 21°C for 45 seconds. The average thickness of the zinc layer on the testing samples was 6 μm.
 Fig. 2 Ecoated aluminium and galvanized steel testing plates.Surface treatment of the testing samples – ecoat
Fig. 2 Ecoated aluminium and galvanized steel testing plates.Surface treatment of the testing samples – ecoat
The testing samples have been ecoated in an ecoat bath at a temperature of 26°C–30°C for 4 minutes, with a chemical ecoat solution by BASF, CathoGuard CG800, and cured at a temperature of 180°C. Figure 2 shows the aluminium and galvanized steel sample after ecoat. The average thickness of the ecoat layer on the testing samples was 17 μm.
Surface treatment of the testing samples – sealing
Three testing samples have been taken out of the set of testing samples after ecoat. A Teroson 1797 sealer, produced by the Henkel company, was applied on the rest of the testing samples with the dimensions of approximately 8 mm (height) × 10 mm (width).
The sealant was intentionally damaged on three testing samples. All the testing samples have been cured at 130°C for 8 minutes. Figure 3 shows the testing samples of aluminium and galvanized steel after pre-treatment, ecoat and sealer application with different degrees of damage.
Accelerated cyclic corrosion test of bimetallic joints
 Fig. 3 Ecoated and sealed aluminium and galvanized steel testing plates.The accelerated cyclic test was performed in a CC2600 salt chamber. The temperature and humidity in the climatic salt chamber were constantly monitored by sensors. During testing, the climate chamber ensured a homogeneous distribution of temperature and humidity throughout the salt chamber. The climatic chamber was equipped with nozzles for homogeneous dispersion of salt mist in the space in which the test samples were placed. Salt spray was not recycled. Uni Jet 800050VP nozzles were used to disperse the salt spray. The salt concentration in salt spray was 0.5% ±0.05% in deionized and distilled water. The test specimens were placed in the salt chamber at 15–20° angles.
Fig. 3 Ecoated and sealed aluminium and galvanized steel testing plates.The accelerated cyclic test was performed in a CC2600 salt chamber. The temperature and humidity in the climatic salt chamber were constantly monitored by sensors. During testing, the climate chamber ensured a homogeneous distribution of temperature and humidity throughout the salt chamber. The climatic chamber was equipped with nozzles for homogeneous dispersion of salt mist in the space in which the test samples were placed. Salt spray was not recycled. Uni Jet 800050VP nozzles were used to disperse the salt spray. The salt concentration in salt spray was 0.5% ±0.05% in deionized and distilled water. The test specimens were placed in the salt chamber at 15–20° angles.
- 6-hour wetting phase, at 25°C ±2°C with salt spray (0.5% NaCl);
- 5-hour drying phase;
- –15.5-hour phase of constant temperature and humidity (T = 50 ±1°C, φ = 70 ±3%).
Results and Discussion
 Fig. 4 Aluminium and galvanized steel testing sample after corrosion test.After 6 weeks of cyclic corrosion testing, the testing samples have been taken out of the climatic chamber and dried.
Fig. 4 Aluminium and galvanized steel testing sample after corrosion test.After 6 weeks of cyclic corrosion testing, the testing samples have been taken out of the climatic chamber and dried.
Testing samples of bimetallic joints without surface treatment
Galvanic corrosion has been detected on the testing samples made of aluminium and galvanized steel. This corrosion took place mainly at the joints of the two materials, as can be seen in Fig. 4. The galvanized steel part was electronegative, and the aluminium part was electropositive. Corrosion developed mainly in the rivet area and also around the edges of the testing plates.
The microscopical analysis has been done. The corrosion process has been spread through the testing sample part made of galvanized steel, as shown in Fig. 5.
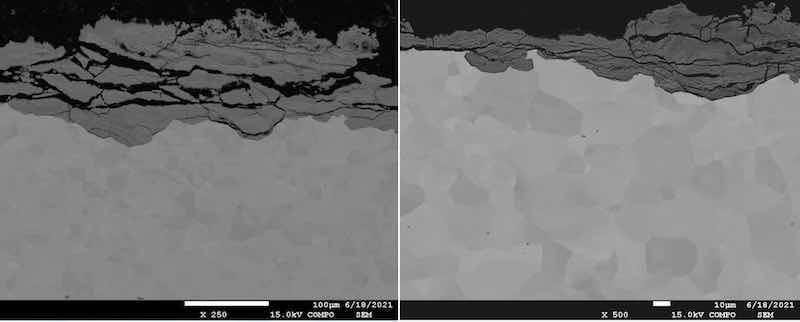 Fig. 5 Microscopical analysis of galvanized steel: a) magnification 250×; b) magnification 500×.
Fig. 5 Microscopical analysis of galvanized steel: a) magnification 250×; b) magnification 500×.
The oxidation process and layer significant degradations have been detected along the grain boundaries, which resulted in peeling of the oxidized parts of the sample. Energy dispersive spectroscopy (EDS) was performed. It is a quantitative analysis of samples used to detect the chemical elements present, as shown in Fig. 6.
 Fig. 6 Energy dispersion spectroscopy of galvanized steel, magnification 750×.The EDS analysis demonstrated that the iron element has been fully present in measuring point 6. Measuring point 5 already contained both the iron element and oxygen element, as an oxide layer had already been formed there. Oxygen measured in measuring point 5 was up to 42%. Measuring point 5 did not contain zinc, so it is possible to conclude that corrosion penetrated only under the protective layer of galvanized steel and devalued it.
Fig. 6 Energy dispersion spectroscopy of galvanized steel, magnification 750×.The EDS analysis demonstrated that the iron element has been fully present in measuring point 6. Measuring point 5 already contained both the iron element and oxygen element, as an oxide layer had already been formed there. Oxygen measured in measuring point 5 was up to 42%. Measuring point 5 did not contain zinc, so it is possible to conclude that corrosion penetrated only under the protective layer of galvanized steel and devalued it.
According to EDS, measuring point 4 contained a smaller part of the zinc element, approximately 4.4%. An increased content of the zinc element, as an element used for galvanizing steels, was located closest to the surface, in measuring points 3, 2 and 1. A relatively high oxygen content was found in measuring points 1, 2, 3, 4 and 5. A significant damage of testing sample surface by galvanic corrosion has been detected, as shown in Table 1.
Table 1: EDS of the galvanized steel testing sample in weight %
| Measuring Point | o2 | fe | Zn | Total |
| 1 | 31.38 | 47.33 | 21.29 | 100 |
| 2 | 37 | 5.17 | 57.83 | 100 |
| 3 | 28.2 | 19.14 | 52.65 | 100 |
| 4 | 33.31 | 62.27 | 4.42 | 100 |
| 5 | 42.04 | 57.96 | 0 | 100 |
| 6 | 0 | 100 | 0 | 100 |
| Maximum | 42.04 | 100 | 57.83 | |
| Minimum | 28.2 | 5.17 | 4.42 |
The chemical map of the elements is shown in Fig. 7. The individual chemical elements were color-coded for better clarity. Carbon was marked in red, oxygen in green, zinc in light green, silicon in light blue, and iron was marked in yellow. Carbon was detected in the marginal area of the sample. This element is contained in the metallographic resin, which was used for encapsulation of the cut sample under microscopic examination.
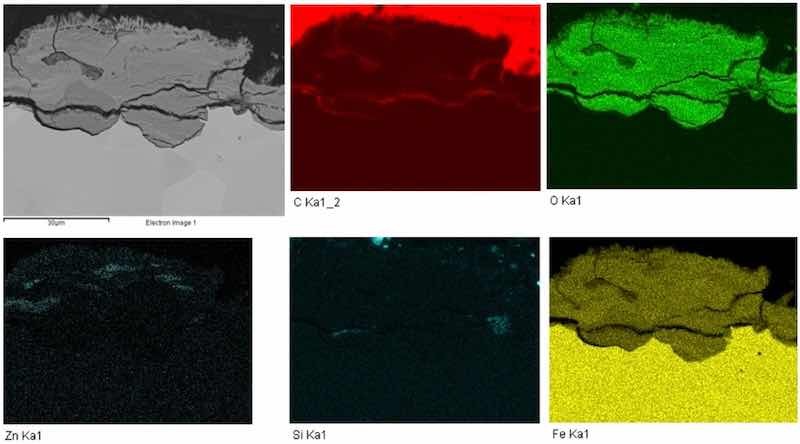 Fig. 7 Cross-section chemical map of elements, galvanized steel, magnification 1,500×.
Fig. 7 Cross-section chemical map of elements, galvanized steel, magnification 1,500×.
Zinc was detected mainly on the surface of the part, which formed a galvanized layer. The distribution of oxygen marked in green was mainly on the surface, where corrosion processes were most extensive. Corrosion expanded along the surface and around the grain boundaries, where the protective zinc layer has been peeled off. Iron element distribution, as shown in yellow, was most extensive in the inner part of the sample and decreased towards the surface. Iron together with oxygen formed an oxide layer on the surface.
Testing samples of bimetallic joints ecoated and PVC (Polyvinil Chloride) sealed
There was no corrosion detected on the ecoated testing plates of bimetallic joints. The testing samples shown in Fig. 8 have been ecoated and PVC sealed. On some of the testing samples, the PVC sealer has been intentionally damaged. It is clear from the figures that there was no corrosion on the testing samples or on the bimetallic joints.
 Fig. 8 Ecoated and PVC sealed aluminium and galvanized steel testing samples after corrosion test.
Fig. 8 Ecoated and PVC sealed aluminium and galvanized steel testing samples after corrosion test.
The evaluation of corrosion testing results was performed according to EN ISO 4628-2:2016. The density of defects and their size were assessed according to Fig. 9. The density of defects at bimetallic joints without surface treatment was rated 5, dense evaluated according to EN ISO 4628-2:2016, because the entire joint was corroded.
The dimensions of individual corrosion defects ranged from 0.5 mm to 5 mm; therefore, the size of defects was rated 4 according to EN ISO 4628-2:2016. Table 2 describes the evaluation of blistering results for the bimetallic testing samples. In total, 10 testing samples of bimetallic combination with different types of surface treatments were evaluated. Figure 9 describes the content of chemical elements in the galvanic steel sample.
Table 2: Corrosion assessment according EN ISO 4628-2:2016
| Type of surface treatment | A | A | A | A | B | B | B | C | C | C |
| Testing sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Defect density | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Defect size | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
A: No surface treatment Ecoated bimetallic joints; B: Ecoated and sealed bimetallic joints; C: Ecoated and sealed bimetallic joints damaged
Discussion and analysis of observation according to EN ISO 4628-1:2016
The evaluation of cyclic corrosion tests consisted of determination of the organic surface resistance of dried painted film. The formation of blisters is one of the first signs of a failure of the film protective function, and also the beginning of the degradation corrosion process, as described in EN ISO 4628-1:2016. After accelerated cycled corrosion tests in the salt chamber with a cyclic atmosphere, alternating wetting phase, drying phase, constant temperature and humidity phase, no visual external corrosion was recorded on the samples where the protective anti-corrosion sealant was used, even in case this application was intentionally, and several times damaged for the evaluated samples of bimetallic joints.
Galvanic corrosion was not detected also on ecoated bimetallic samples, corrosion was found neither on samples nor on joints. Extensive corrosion has been detected on bimetallic joints without any surface treatment. In case this accelerated cyclic corrosion test is continuing further, a significant decrease of quality and degradation of the testing sample and bimetallic joints is estimated. The galvanized steel part of the testing sample corroded, and its quality started to degrade. On the other hand, the aluminium part of the testing sample did not corrode and held its previous properties.
Discussion and future research
Cyclic corrosion testing and testing samples preparation is relatively time consuming, not just because it is necessary to wait for 6 weeks for the results, but due to the correct technological and process sequence as well. On the other hand, the evaluation of corrosion tests is relatively simple and quick. The corrosion degradation of testing sample is visually verified. Results are visible as soon as the tests are completed. The approach of testing samples preparation used in this particular article is unique and unpublished. This method of performing corrosion tests is closest to galvanic corrosion in the real environment, it is using a combination of exact same materials and technological processes as in real production, and cyclic corrosion testing simulates changing environmental properties in surrounding areas.
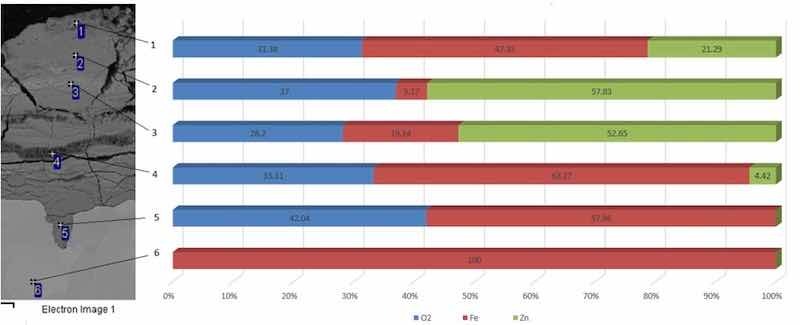 Fig. 9 The graph of chemical elements content in weight %, galvanized steel sample.
Fig. 9 The graph of chemical elements content in weight %, galvanized steel sample.
Most of the corrosion tests are performed and focused on individual particular materials and are performed separately. As an example, Votava et al. (2018) deal with monitoring the corrosive degradation rate of zinc and duplex coatings usable in technical practice as anti-corrosion protection in the tanks for transportation (Votava et al., 2018). Another example of corrosion testing approach is described by Lozrt et al. (2021), where the testing samples of hot-dip galvanized steel are subjected to an intact corrosion resistance test in a salt spray environment.
A similar method of performing cyclic corrosion testing is described by Allély et al. (2014) – the testing of bimetallic joints of aluminium-coated boron and galvanic steel is subjected to cyclic corrosion testing according to VDA 233-102. In this case, however, it is joining of two bimetallic materials with an adhesive tape. The testing samples are not mechanically joined by nondetachable joints. Another interesting method of galvanic corrosion testing is published by Feng and Frankel (2014), but only one type of test material is tested against screwed hexagonal screws from another material. Galvanic corrosion behaviour of dissimilar stainless steels is described by Zhou et al. (2021), where stainless steel 420 is welded with austenitic steel 304L. Galvanic corrosion examination on testing plates made of 99.5% titanium and 99.5% copper joined by epoxy resin is described by Xavier (2020).
There are also different tests to evaluate the ability of corrosion systems, for example, bending test, pull-off test, as mentioned by Votava et al. (2020). The method and way of sample preparation, mechanical joining of two types of different materials by riveting, or other mechanically inseparable joint, and surface treatment exactly according to the technological processes used in the automotive industry have not been published widely in scientific articles. This method has not been standardized by internationally valid standards as well. The shift of the automotive industry to electric vehicles impacted the car design significantly, with an increased use of bimetallic joints made of different materials. Based on testing sample preparation and corrosion testing published in this article, it is recommended to create an international standard for cyclic corrosion testing for bimetallic joints of electrical vehicles.
Conclusion
The performed cyclic corrosion testing of bimetallic joints in a salt chamber confirmed the presence of galvanic corrosion in case of a bimetallic joint without surface treatment. Based on visual evaluation according to EN ISO 4628-2:2016, defect density was evaluated with score 5. The dimensions of individual corrosion defects were evaluated according to EN ISO 4628-2:2016 as scored 4, with dimensions from 0.5 mm to 5 mm.
The microscopic and energy-dispersive analysis of the bimetallic sample without surface treatment also confirmed the presence of galvanic corrosion with a wider oxygenation process on the surface. From measuring point 1 up to measuring point 5, the extreme penetration of oxides into the surface of the corroded galvanized steel sample material was recorded. The protective layer of zinc galvanized steel did not prevent this penetration either. Based on the visual evaluation according to EN ISO 4628- 2:2016, the defect density on all other samples 2–10 was evaluated with score 0, and the defect size evaluated with rating 0 was invisible.
The corrosion test and the subsequent visual, microscopic and energy dispersive analysis of the samples proved that galvanic corrosion did not occur in ecoated testing samples, ecoated and PVC sealed testing samples, and ecoated testing samples with damaged PVC sealing. Cyclic corrosion testing in the salt chamber for 6 weeks has clearly proved that the most important surface treatment to prevent the galvanic corrosion of bimetallic joints is ecoat (cataphoresis).
The ecoat layer sufficiently separates materials with different electro-potentials, so it is an adequate surface treatment against the galvanic corrosion of bimetallic joints for at least 6 years of service life, if not mechanically damaged. The further development of surface treatment and anti-corrosion systems will include the sustainability approach, low bake ecoat solutions, CO2 reduction to zero, natural gas elimination in curing processes, and reductions of multilayer systems, along with the advanced joining methods effect and suitable surface treatment selection.
Acknowledgments: This publication was supported by KEGA project no. 003SPU-4/2021 “Innovation of Study Programmes Using New Methods of Education and Progressive Manufacturing Technologies.”
Authors Ján Lilko, Martin Kotus, Martin Baráth, and Róbert Drlička are with the Slovak University of Agriculture in Nitra, Faculty of Engineering, Nitra, Slovak Republic.
References
- ALLÉLY, C. L. – DOSDAT, L. – CLAUZEAU, O. – OGLE, K. – VOLOVITCH, P. 2014. Anticorrosion mechanisms of aluminized steel for hot stamping. In Surface and Coatings Technology, vol. 238, pp. 188–196.
- ASTM-B117-19: 2019. Standard practice for operating salt spray (fog) apparatus.
- BRITNER, A. – DIEU, C. – PODLESCHNY, R. 2021. Corrosion protection for cold-formed structural steel elements. In Steel Construction, vol. 14, no. 4, pp. 250–257.
- CISCHINO, E. – VULUGU, Z. – EZEIZA, C. E. – BENITO, I. L. – MANGINO, E. – CRISTIANSEN, J. C. – SANPOREAN, C. G. – PAOLO, F. – KIRPLUKS, M. – CABULIS, P., 2017. A concrete and viable example of multi- material body: The Evolution project main outcomes. In Proceedia CIRP, vol. 66, pp. 300–305.
- EN ISO 4628-1:2016. Paints and varnishes – Evaluation of degradation of coatings – Designation of quantity and size of defects, and of intensity of uniform changes in appearance – Part 1: General introduction and designation system.
- EN ISO 4628-2:2016. Paints and varnishes – Evaluation of degradation of coatings – Designation of quantity and size of defects, and of intensity of uniform changes in appearance – Part 2: Assessment of degree of blistering.
- EN ISO 9227:2017. Corrosion tests in artificial atmospheres – Salt spray tests.
- EN ISO 12944-1:2017. Paints and varnishes – Corrosion protection of steel structures by protective paint systems – Part 1: General Introduction.
- FENG, Z. – FRANKEL, G. S. 2014. Galvanic test panels for accelerated corrosion testing of coated Al alloys: Part 2 – Measurement of galvanic interaction. In Corrosion, vol. 70, no. 1, pp. 95–106. FENTAHUN, M. A. – SAVAS, M. A. 2018. Materials used in automotive manufacture and material selection using Ashby charts. In International Journal of Materials Engineering, vol. 8, no. 3, pp. 40–54.
- GOLDSCHMIDT, A. – STREITBERGER, H. J. 2007. Basics of Coating Technology. 2nd ed., Hannover : Vincentz Network, 792 pp. ISBN 9783866309036.
- GULLINO, A. – MATTEIS, P. – D’AIUTO, F. 2019. Review of aluminium- to-steel welding technologies for car-body applications. In Metals, vol. 9, no. 3, article no. 315.
- LIU, M. – GUO, Y. – WANG, J. – YERGIN, M. 2018. Corrosion avoidance in lightweight materials for automotive applications. In Materials Degradation, vol. 2, article no. 24.
- LOZRT, J. – VOTAVA, J. – ŠMAK, R. 2021. Duplex anti-corrosion protection of steel using a combination of hot-dip galvanising and water-soluble paints. In Acta Technologica Agriculturae, vol. 24, no. 3, pp. 129–135.
- REIF, K. 2014. Fundamentals of Automotive and Engine Technology. Wiesbaden : Springer Vieweg, 286 pp. ISBN 978-3-658-03972-1. TPJLR 52.265. Laboratory accelerated cyclic corrosion tests. Jaguar Land Rover.
- VDA 233-102: 2013. Cyclic corrosion testing of materials and components in automotive construction. Verband der Automobilindustrie.
- VOTAVA, J. – JUKL, M. – POLCAR, A. – KUMBÁR, V. – DOSTÁL, P. 2018. Anti-corrosion systems in vehicles for the transportation and application of fertilizers. In Metallic Materials, vol. 56, no. 2, pp. 131–136.
- VOTAVA, J. – KUMBÁR, V. – POLCAR, A. – FAJMAN, M. 2020. Change of mechanical properties of zinc coatings after heat treatment. In Acta Technologica Agriculturae, vol. 23, no. 1, pp. 7–11.
- XAVIER, J. R. 2020. Galvanic corrosion of copper/titanium in aircraft structures using a cyclic wet/dry corrosion test in marine environment by EIS and SECM techniques. In SN Applied Sciences, vol. 2, article no. 1341.
- ZHOU, Y. – QI, J. – ENGELBERG, D. L. 2021. On the application of bipolar electrochemistry for simulating galvanic corrosion behaviour of dissimilar stainless steels. In Electrochemistry Communications, vol. 126, article no. 107023.



































