Aerospace coatings for exterior applications require a demanding set of performance attributes to provide acceptable performance from both a functional and aesthetic standpoint.
 Ron LewarchikIn many cases the cost of a new commercial aircraft can be over $300 million with the expectation of lasting several decades with flight times of 4,000 hours or more on an annual basis. According to GMI, the aerospace coating market size is estimated to surpass $1 Billion in sales by 2024.
Ron LewarchikIn many cases the cost of a new commercial aircraft can be over $300 million with the expectation of lasting several decades with flight times of 4,000 hours or more on an annual basis. According to GMI, the aerospace coating market size is estimated to surpass $1 Billion in sales by 2024.
Aerospace coating requirements can include:
- Ability to maintain adhesion and flexibility when subject to rapid temperature changes from 120F to – 70F in a matter of a few minutes
- Resistance to hydraulic fluids including Skydrol, diesel fuel, lubricating oils and deicing fluids
- Resist degradation when exposed to intense UV light at high altitudes
- Repeated dry hot and cold moist cycles
- Outstanding corrosion resistance as aircraft are often operated in marine and industrial environments
- High degree of flexibility and resistance to stress as a result of turbulence, vibration and wing flexing
- Abrasion and erosion resistance and paint from dirt and sand at sub and supersonic speeds
- Infrared (IR) reflectivity (military applications)
- Low density (offers weight savings)
- Icephobic
- Low COF
The substrate for the fuselage and aircraft skin is predominantly AA 2024 aluminum. AA 2024 is an alloy of copper and aluminum. The copper provides an increase in the strength to weight relationship, however it is also detrimental to corrosion resistance. Weight reduction is an enormous driving force in new aircraft design as it equates to fuel savings, speed and range. Composites, fiber metal laminates and aluminum-lithium alloys are being used on an increasing basis.
A number of cleaning/pretreatment types (historically hexavalent chrome-based) provide a thin protective layer to improve corrosion resistance as well as receptivity of subsequent coats as it increases surface tension and polarity of the surface.
- Organic Coatings typically include a primer, pigmented basecoat and a clearcoat.
- Primers are typically organic solvent borne and waterborne two-component epoxy-polyamine/polyamide types containing extenders, additives, catalysts and are further fortified with corrosion inhibitive pigments.
Common types of corrosion on aircraft include filiform, pitting, intergranular, exfoliation, stress cracking, galvanic and crevice corrosion. All these types of corrosion are exacerbated by moisture, salt, thermocycling and direct contact of metals differing in metallic content.
Common corrosion inhibitive pigments historically used in aerospace primers include barium chromate and strontium chromate. Epoxy resins for the most part are combinations of bisphenol A and bisphenol F types. When formulated with suitable crosslinking agents (normally amine or amido-amine type) epoxy-based primers provide excellent adhesion, corrosion resistance and chemical resistance.
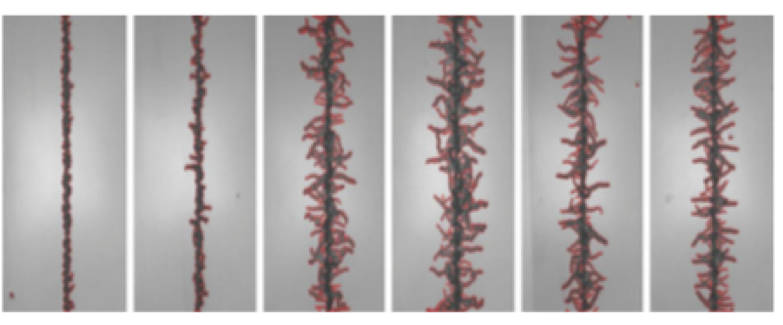
Figure 1. Images of Filiform Corrosion on Coated Aluminum
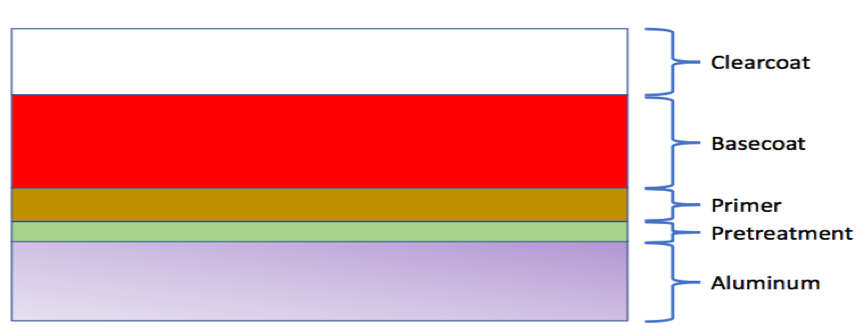
Figure 1a. Cross-section of Aerospace Coating Layers
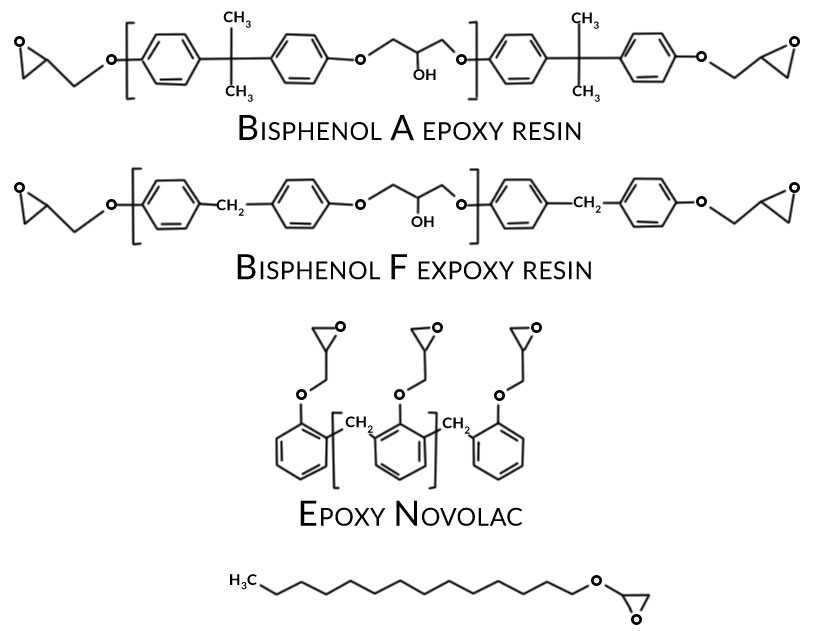
Figure 2. Typical epoxy resins and epoxy functional reactive diluents used in aerospace primers
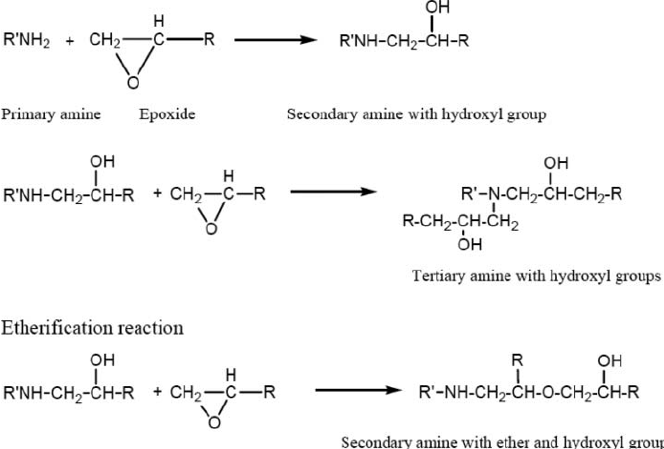
Figure 3 Reactions of epoxy resins with amino functionalities
Aerospace exterior topcoats are two-component urethane types comprised of hydroxyl functional resins [polyesters, acrylics or fluorinated ethylene vinyl ethers (FEVE)] reacted with isocyanate prepolymer(s). Typical curing reactions are as follow:
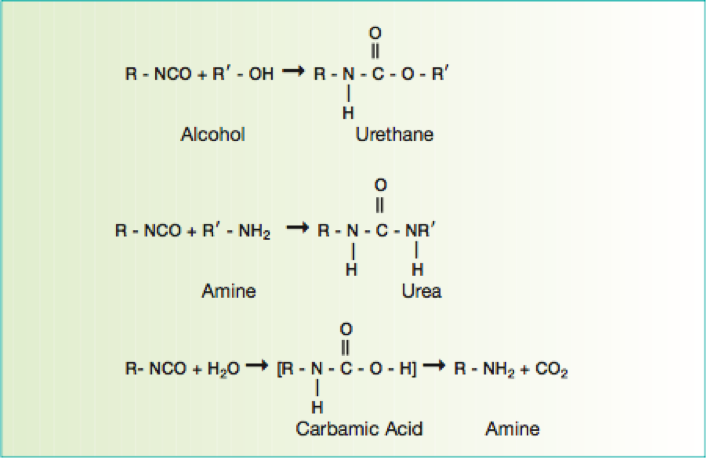
Figure 4 Reactions of polyols with isocyanate functional cross-linkers
Due to the demanding requirements of aerospace coating systems, chemists use a stoicheometric excess of isocyanate crosslinker to provide excellent chemical resistance. The excess isocyanate crosslinker reacts with moisture to decarboxylate to form a polyurea upon further reaction. Typically a 50 percent or more stoichiometric excess of isocyanate is used to ensure a high degree of polyurea formation.
Polyureas are known for their superb resistance to aggressive fluids such as Skydrol (an aircraft hydraulic fluid). Polyester polyols are used primarily in the pigmented basecoat portion of the two component polyurethane coating, whereas acrylic polyols and also FEVE-based polyols are primarily used in the clearcoat portion of the polyurethane topcoat.
Clearcoats are further fortified with both UV absorbers as well as hindered amine light stabilizers to further protect the coating system from degradation due to exposure to intense upper atmosphere UV light.
Isocyanate crosslinkers are typically derived from hexmethylene diisocyante (HMDI) and/or isophorone isocyanate (IPDI). The former type provides flexibility, whereas the latter can provide improved hardness.
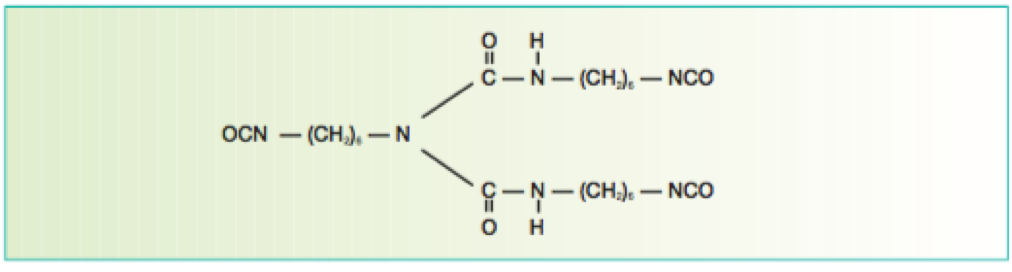
Figure 5 Biuret formed from the reaction of three HMDI molecules
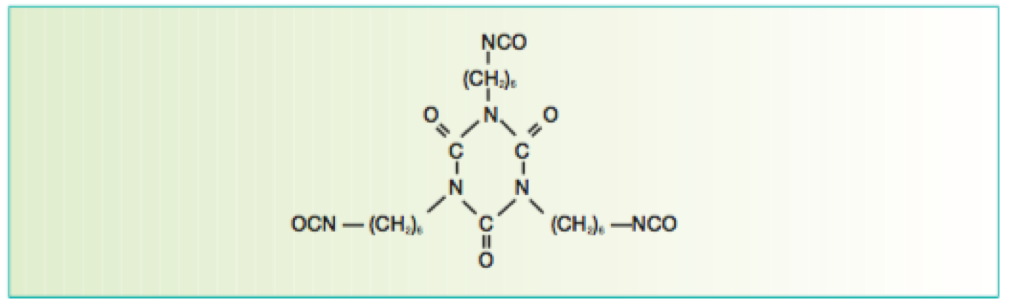
Figure 6 Isocyanurate formed from the reaction of three HMDI molecules
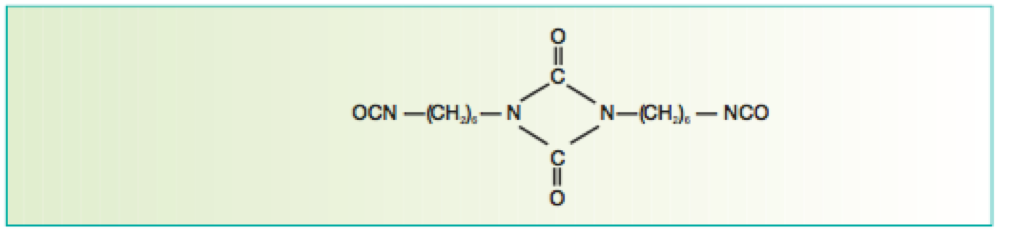
Figure 7 Uretdione formed from two HMDI molecules
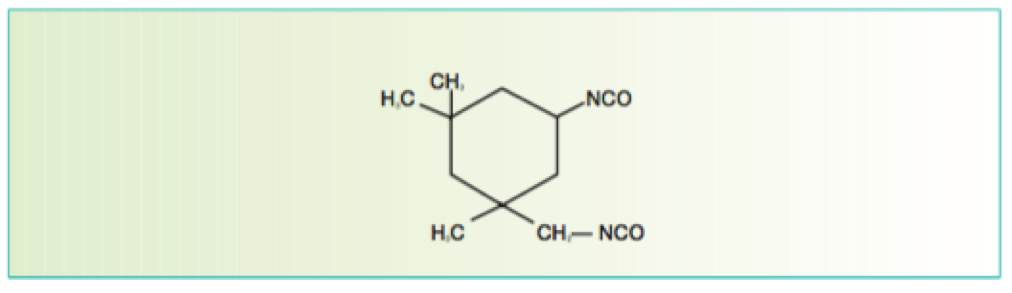
Figure 8 Isophorone Diisocyanate
Isocyanurate-based isocyanate cross linkers provide excellent weathering characteristics when reacted with a suitable polyol resin system and are thus widely used in aerospace topcoats.
Recent innovations and project emphasis in aerospace coatings include chrome-free pretreatment-primers and chrome-free epoxy primers. Drag-reducing topcoats that provide a 1 percent improvement in fuel efficiency can lower fuel costs by $700 million a year, according to the International Air Transport Association (IATA). On average, airlines incur about $100 a minute per flight in total operating costs, IATA says. Therefore, even saving just one minute of flight time could reduce total industry operating costs by more than $1 billion a year and significantly reduce environmental emissions.
References:
- Active Protective Coatings, Springer et.al., 2016
- Organic Coatings Science and Technology, 3rd Edition, Wicks et.al, 2007
Ronald J. Lewarchik, President and CEO of Chemical Dynamics, LLC, brings 40 years of paint and coatings industry expertise to his role as a contributing author with the Prospector Knowledge Center. As a contributing writer, Ron pens articles on topics relevant to formulators in the coatings industry. He also serves as a consultant for the Prospector materials search engine, advising on issues related to optimization and organization materials within the database. Visit http://www.chemicaldynamics.net/



































