Due to the growing interest in electromobility and the increasing use of electrical contacts for sensors and electrical components, the demand for reliable electrical plug contacts is increasing.
These are subjected to mechanical stresses caused by friction and temperature loads with a simultaneous requirement for low contact resistance. Silver is the material of choice due the best electrical properties. This can be significantly improved in its application-relevant properties by alloying or using dispersion materials. Long-term stability at temperatures of up to 200°C can be ensured with the new coating system consisting of hard silver with antimony as an alloying element and graphite as a dispersion material. It also has excellent friction values and low contact resistance. These characteristics have been proven both on test benches and in vehicle-related test environments.
BatteryElectric Vehicles Reach Record Figures
The market share of battery-electric vehicles also reached new record figures in 2023, driven in particular by record sales in China [1]. This is very likely to continue in 2024 and beyond. This rapidly advancing market penetration in large parts of the world and the associated rapid technical progress in the field of electromobility and automated driving are leading to a sharp increase in the requirement of electronic components in vehicles. In the field of silver-based electrical connector systems, these manifest in increasing mechanical loads featuring a high number of mating cycles and a shift in the temperature load collective – individually or in combination– with a simultaneous requirement of low electrical contact resistance. Specific requirements for the silver coating can vary depending on the area of application:
Even in combustion engine cars there are applications arising where specific connectors face requirements with up to 50 mating cycles at a currently targeted temperature load of approx. 180°C. In the area of charging socket the requirement is for up to around 10,000 mating cycles at a maximum temperature load of 150°C [2]. Higher temperature loads are also expected here in the future.
Fine silver coatings are not physically capable to fulfil this multitude of different conditions at the same time. Why is this? Fine silver has the distinct property of cold welding [3]. This can be a welcome feature for certain applications, such as press fits, but completely undesirable for detachable connectors with a high number of mating cycles. In addition, silver and copper show a very pronounced diffusion behavior into each other, so a diffusion barrier is required for electrical contacts [3]. This is typically achieved by a thin intermediate nickel layer. However, this creates another problem: the delamination of the silver layer from the nickel diffusion barrier in the temperature range above 150°C. This is caused by the formation of nickel oxide at the silver nickel interface due to a pronounced diffusion behavior of oxygen in silver at temperatures above 160°C [3]. The high demands placed on electrical connectors and the physical properties of silver create a great need for development so that it is possible to offer distinct, cost-efficient solutions for these problems described above, avoiding the usage of cost driving other precious metals such as palladium.
SilverGraphite Dispersion Layers for Sliding Contacts
In this context, silver graphite dispersion layers for sliding contacts in medium and high voltage systems have been developed over many decades and have been adapted and optimized over the years to meet new technical requirements [47]. The graphite particles being incorporated into the silver layer ensure a significant reduction in the friction coefficient of the layer due to their lubricating properties and, thus, also prevent the effect of cold welding known from fine silver. In this way, the requirement for a high number of mating cycles can be achieved simultaneously with a low contact resistance and high electrical conductivity.
However, the fulfillment of the requirement for high-temperature stability of the coating remained unresolved till now. This requirement is solved by introducing a diffusion barrier between the nickel and silver graphite layer. This diffusion barrier consists of a functional hard silver layer containing antimony. In principle, other alloying elements such as Se, In, Te, Bi, or similar elements or combinations thereof are also suitable for this purpose to effectively reduce diffusion paths for oxygen in silver or to greatly impede diffusion.
The results of such a coating sequence with an antimony-containing hard silver intermediate layer and silver graphite as the final layer (SLOTOCONNECT HT 4200 CF) or with fine silver passivated with ODT as the final layer (SLOTOCONNECT HT 4200) and without an antimony containing hard silver intermediate layer and silver graphite as the final layer (SLOTOSIL SG1910) are presented below. As series reference, fine silver passivated with ODT is used. The corresponding layer sequences are plated onto nickel-plated copper test sheets with approximately 4 µm of antimony containing hard silver and approximately 4 µm of silver graphite, respectively, approximately 4 µm of fine silver as a final layer or only silver graphite with a layer thickness of approximately 8 µm. Nickel-plated copper sheets were used as a reference with a final layer of around 8 µm fine silver. The fine silver end layers were also passivated with ODT. The selected layer thicknesses are exemplarily selected and can also be higher or lower depending on the technical requirements. The layer sequences and coated test specimens are shown in Fig. 1.
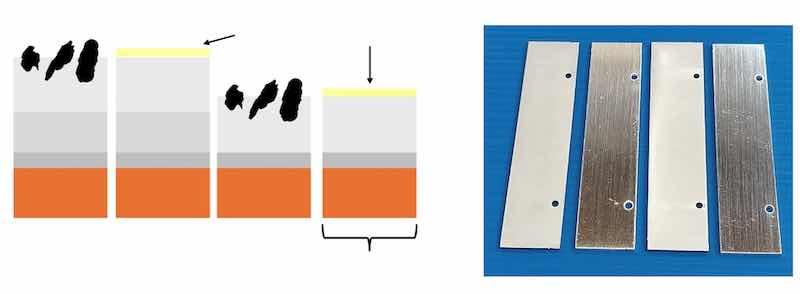
Fig. 1 a) Schematic drawing of the analysed layer sequences of the coating systems, b) Photo of the coated test specimens in the sequence shown in a).
Correlation Between Friction Coefficient
 Fig. 2 Tribological testing rig for measuring the tribological and electrical properties of the coating structures.The coated components were analysed on a physical testing rig (WECOX) developed in cooperation with iChemAnalytics. One of the special features of this test rig is that the contact resistance can also be measured in situ during the cyclical measurement of the friction coefficient so that a cycle or time-resolved direct correlation between the friction coefficient and the electrical contact resistance of the respective surface under investigation can be made. Such measurements can be made on four specimens simultaneously. The corresponding testing rig is shown in Fig. 2.
Fig. 2 Tribological testing rig for measuring the tribological and electrical properties of the coating structures.The coated components were analysed on a physical testing rig (WECOX) developed in cooperation with iChemAnalytics. One of the special features of this test rig is that the contact resistance can also be measured in situ during the cyclical measurement of the friction coefficient so that a cycle or time-resolved direct correlation between the friction coefficient and the electrical contact resistance of the respective surface under investigation can be made. Such measurements can be made on four specimens simultaneously. The corresponding testing rig is shown in Fig. 2.
The characterization of the mating cycle stability is performed on the coated test specimens (see Fig. 1b) with the layer sequenced depicted in Fig .1a.Silver spheres with a diameter of 3 mm are used as test counterparts. The test specimens were tested at 23°C for 1,000 cycles each at
a normal force of 1.5 N, a test frequency of 1 Hz, a deflection amplitude of 500 µm and a test current of 100 mA in the tribological test rig. The corresponding cycle resolved measurement data of the friction coefficient and the electrical contact resistance are shown in Fig. 3. Figure 3 a) shows that the initial friction coefficient of the two test specimens coated with silver graphite as the final layer is initially between 0.1 and 0.2. With increasing cycle number, the friction coefficient rises linearly to around 0.26 after 1,000 test cycles. The fine silver coating systems passivated with ODT initially exhibit a significantly higher friction coefficient of around 1.1, which rises to around 1.3 within the first few cycles, begins to fall after around 30 cycles and reaches around 0.9 (antimony containing hard silver /fine silver coating system) or 1.1 (fine silver coating system) after 400 cycles, being clearly well above the silver graphite coating systems. The test specimens coated with silver graphite show no signs of wear after 1,000 test cycles due to the lubrication by graphite. This can also be seen in the in situ, cycle-resolved measurements of the contact resistance. The two test specimens coated with silver graphite as the final layer show that the initially measured contact resistance is around 1.1 mΩ, decreasing linearly increasing cycle number until it reaches around 0.9 mΩ after 1,000 cycles – similar to the friction coefficient. This means that there is no indication of a significant wear of the coating systems with silvergraphite as final layer, even with respect to electrical contact resistance. The contact resistance of the silver reference system behaves similar with the initial contact resistance dropping from 0.9 mΩ to around 0.8 mΩ.
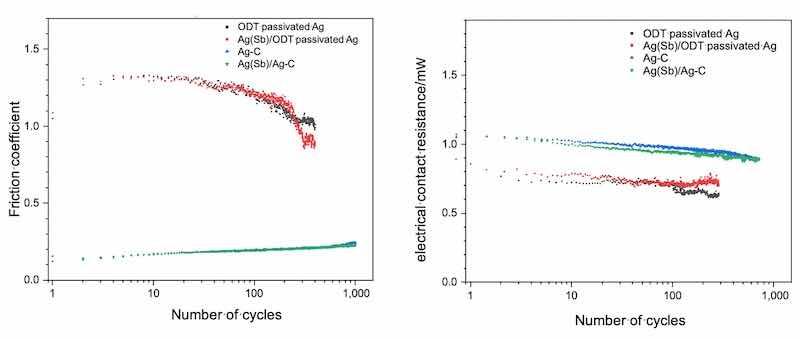
Fig. 3 Connector Mating cycles of the a) friction coefficients and b) electrical contact resistances of the analysed silverbased coating systems.
Stability of Coated Components Under High Temperatures
As described in the beginning, the stability of the coated components under high temperatures is significantly important for the dedicated technical applications. This was investigated as part of temperature ageing tests at 200°C for 1,000 hours with intermediate withdrawals after 200 hours, 400 hours, 600 hours and 800 hours. The adhesive strength of the silver coating systems was assessed by means of a classic bending test on the nickel-plated copper test specimens after temperature exposure. This reveals that the silver coating systems without an antimony-containing hard silver intermediate layer failed after just 600 hours due to local delamination on the nickel layer, see Fig. 4a). The test specimens with layer structures consisting of an antimony containing hard silver intermediate layer and silver graphite as final layer or an antimony containing hard silver intermediate layer and fine silver as final layer, on the other hand, consistently passed the adhesion tests, see Fig. 4b).

Fig. 4. Light micrographs after adhesion test of the test specimens with a a) fine silver layer passivated with ODT and b) layer sequence with the antimony containing hard silver intermediate layer and silvergraphite as final layer.
The results presented here could also be independently reproduced by suppliers from the automotive sector. In summary, the SLOTOCONNECT HT 4200 CF coating system excellently fulfills the technical requirements regarding temperature stability, number of connector mating cycles, and electrical contact resistance as described above and, thus, represents a cost-efficient solution without negatively influencing other requirements placed on a silver-based series connector system. If the properties of fine silver already fulfill the requirements of the coating system in terms of a maximum number of connector mating cycles, the SLOTOCONNECT HT 4200 coating system is the ideal graphite-free solution.
Written by By Vera Lipp, Stefan Henne, and MarkDaniel Gerngroß with the Dr Max Schlötter GmbH & Co. KG, Geislingen
References
[1] N. Carey, Global electric car sales rose 31% in 2023 – Rho Motion, Reuters, 2024.
[2] S. Berger, F. Talgner & R. Ziebart, SilberPalladium Schichten als Kontaktoberflächen, Galvanotechnik,1 p.31 – p.38, Leuze Verlag, 2021.
[3] H. Schmid & I. Buresch, Oberflächen für Steckverbinderkontakte, in Praxishandbuch Steckverbinder (Hrsg H. Endres), p.245–286 Vogel Communications Group, Würzburg, 2021.
[4] G. Clarsbach Behringer, H. Laub & S. Zjilstra, Cyanidischer, Silberelektrolyt und Verfahren zur galvanischen Abscheidung von SilberGraphitDispersionsüberzügen und seine Anwendung 1978.
[5] P. Rehbein & V. Haas, Kontaktoberflächen für elektrische Kontakte, EP1673836B1, 2010.
[6] A. Stadler, R. Sottor, R. Wagner, C. Diandl & S.Heitmüller, Silberelektrolyt zur Abscheidung von DispersionsSilberschichten und Kontaktoberflächen mit DispersionsSilberschichten, EP3797184B1, 2023.
[7] F. Talgner: SilberGraphitBeschichtung als neuer Standard für Steckverbinder in Hochstromanwendungen; WOMag 10/2022; https://www.wotechtechnicalmedia.de/womag/ausgabe/2022/10/08_umicore_agc_10j2022/08_umicore_agc_10j2022.php































