Most energy storage systems (ESSs) have recently adopted lithium-ion batteries (LIBs), with the highest technology maturity among secondary batteries.
 Dr. Minah LeeHowever, these are argued to be unsuitable for ESSs, which store substantial amounts of electricity, owing to fire risks. The instability of the international supply of raw materials to construct LIBs has also emerged as a crucial concern. By contrast, aqueous zinc-ion batteries (AZIBs) use water as the electrolyte, which fundamentally prevents battery ignition. Furthermore, the price of zinc, the raw material, is only one-sixteenth of that of lithium.
Dr. Minah LeeHowever, these are argued to be unsuitable for ESSs, which store substantial amounts of electricity, owing to fire risks. The instability of the international supply of raw materials to construct LIBs has also emerged as a crucial concern. By contrast, aqueous zinc-ion batteries (AZIBs) use water as the electrolyte, which fundamentally prevents battery ignition. Furthermore, the price of zinc, the raw material, is only one-sixteenth of that of lithium.
The research team led by Dr. Minah Lee at the Energy Storage Research Center in the Korea Institute of Science and Technology (KIST; President Seok-Jin Yoon) announced that they had succeeded in developing a technology for manufacturing “high-density zinc metal anodes,” which is key to commercializing AZIBs. This manufacturing technology is expected to act as a catalyst for the mass production of AZIBs because zinc metal anodes with high energy density and long lifespan can be produced through a simple electroplating process by using low-cost and ecofriendly solutions.
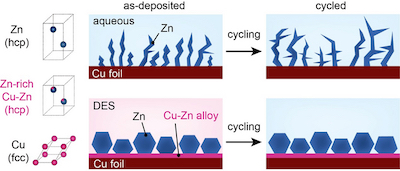 Unlike zinc particles, which are irregularly formed in a conventional aqueous electrolyte and induce corrosion, zinc grown in a DES solution is tight and uniform and maintains a stable structure even after charging and discharging in an aqueous electrolyte. (KIST)In theory, because AZIBs utilize two electrons per ion, they are advantageous in terms of volumetric energy density relative to alkali metal-ion batteries. If the capacity of the zinc metal used as the anode for making the battery does not exceed twice that of the cathode, it is possible to realize an energy density comparable to that of the LIBs commercialized today. Furthermore, even if the capacity of the zinc metal reaches five times that of the cathode, it is still competitive in that it is similar to that of sodium-ion batteries, which are attracting attention as the next generation of batteries owing to their low cost and material abundance.
Unlike zinc particles, which are irregularly formed in a conventional aqueous electrolyte and induce corrosion, zinc grown in a DES solution is tight and uniform and maintains a stable structure even after charging and discharging in an aqueous electrolyte. (KIST)In theory, because AZIBs utilize two electrons per ion, they are advantageous in terms of volumetric energy density relative to alkali metal-ion batteries. If the capacity of the zinc metal used as the anode for making the battery does not exceed twice that of the cathode, it is possible to realize an energy density comparable to that of the LIBs commercialized today. Furthermore, even if the capacity of the zinc metal reaches five times that of the cathode, it is still competitive in that it is similar to that of sodium-ion batteries, which are attracting attention as the next generation of batteries owing to their low cost and material abundance.
However, zinc metal anodes restrict the energy density and lifespan of AZIBs because of the irregular growth of nanoparticles during battery operation. A low zinc metal particle density and a large surface area in the anode accelerate corrosion with the electrolyte, thus depleting the active zinc metal and the electrolyte. Existing studies have typically used zinc metals that were 20 times thicker than what was required to counteract the lifespan limitations; paradoxically, this led to an inevitable decline in energy density and cost competitiveness, the biggest strengths of AZIBs.
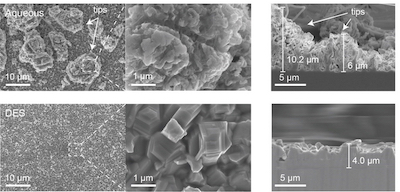 Microstructure of the surface and cross-section after electroplating of zinc. (KIST)Thus, the team led by Dr. Minah Lee at the KIST controlled the microstructure of zinc metal anodes to reduce the prevalence of the side reactions that induce the decline in energy density and lifespan of AZIBs. The team adopted a deep eutectic solvent (DES) solution, which can be easily synthesized at room temperature, was to construct the compact zinc anodes. This DES solution is composed of choline chloride and urea mixed at a mole ratio of 1:2; the mixture becomes a liquid complex with a melting point of 12°C. The researchers confirmed that a zincophilic copper–zinc alloy layer spontaneously forms between the zinc and copper current collectors within the DES, enabling high-density zinc particles to grow. The researchers succeeded in using this discovery to develop an electroplating process that allows zinc metals to grow densely and evenly in the low-cost and ecofriendly DES solution.
Microstructure of the surface and cross-section after electroplating of zinc. (KIST)Thus, the team led by Dr. Minah Lee at the KIST controlled the microstructure of zinc metal anodes to reduce the prevalence of the side reactions that induce the decline in energy density and lifespan of AZIBs. The team adopted a deep eutectic solvent (DES) solution, which can be easily synthesized at room temperature, was to construct the compact zinc anodes. This DES solution is composed of choline chloride and urea mixed at a mole ratio of 1:2; the mixture becomes a liquid complex with a melting point of 12°C. The researchers confirmed that a zincophilic copper–zinc alloy layer spontaneously forms between the zinc and copper current collectors within the DES, enabling high-density zinc particles to grow. The researchers succeeded in using this discovery to develop an electroplating process that allows zinc metals to grow densely and evenly in the low-cost and ecofriendly DES solution.
Application of the manufactured zinc metal anode to an aqueous zinc battery system showed that the corrosion reactions are effectively suppressed, and the capacity is maintained at more than 70% after more than 7000 repeated charges and discharges. This result is exceptional relative to those of similar existing studies that utilized thin zinc, and the values far exceed the charging and discharging lifespans (1000–2000 times) of commercial LIBs.
“We were able to develop a core technology for commercializing AZIBs that can solve the fire safety issue of ESSs, which is the biggest obstacle to the provision and expansion of renewable energy.” Dr. Lee says. “We expect that this compact zinc anode manufacturing technology will open the way for the mass production of AZIBs by combining a particularly economical and ecofriendly DES solution with an electroplating process already widely used throughout the industry.”
KIST was established in 1966 as the first government-funded research institute in Korea to establish a national development strategy based on science and technology and disseminate various industrial technologies to promote the development of major industries. KIST is now elevating the status of Korean science and technology through the pursuit of world-leading innovative research and development. For more information, please visit KIST’s website at https://eng.kist.re.kr/
This study was supported by the Ministry of Science and ICT (Minister Jong-Ho Lee), and was conducted through the Nano·Future Material Original Technology Development Program and the Mid-careear Researcher Program of the National Research Foundation and the KIST institutional program. The research results have been published in the latest online edition of Energy & Environmental Science (IF: 38.532, top 0.182% in the JCR field), a prestigious journal in the field of energy and environmental science.



































