For more than 50 years, aluminum-ceramic coatings have played an essential role in protecting steel components from heat and corrosion in turbines and turbomachinery.
 Bruce McMordieThese aqueous “spray and bake” slurries combine the aluminum powder with phosphoric and chromic acid (chromium trioxide). These slurries contain hexavalent chromium (Cr+6), which is a known carcinogen. Since 1990, the industry has sought a Cr-Free alternative with comparable performance. That quest has been plagued by false starts and dead ends, unexpected setbacks that revealed how much needed to be learned and unlearned.
Bruce McMordieThese aqueous “spray and bake” slurries combine the aluminum powder with phosphoric and chromic acid (chromium trioxide). These slurries contain hexavalent chromium (Cr+6), which is a known carcinogen. Since 1990, the industry has sought a Cr-Free alternative with comparable performance. That quest has been plagued by false starts and dead ends, unexpected setbacks that revealed how much needed to be learned and unlearned.
This article reviews the history of these uniquely capable Al-ceramic protective coatings and efforts to develop Cr-free alternatives and explains the promise of combining slurries of different, safer chemistries in a novel way to produce an aluminum-ceramic coating that is protective yet non-hazardous.
An Urgent Need
 FIGURE 1: Aluminum powder particles cemented together by chromate/phosphate binder protects steels and other alloys from oxidation and corrosion up to 1000oF (538oC).In the 1950s, the development of flight gas turbine engines was hindered by a lack of coatings to protect steel from salt corrosion and heat. No existing paint could survive the temperatures seen in these new powerplants.
FIGURE 1: Aluminum powder particles cemented together by chromate/phosphate binder protects steels and other alloys from oxidation and corrosion up to 1000oF (538oC).In the 1950s, the development of flight gas turbine engines was hindered by a lack of coatings to protect steel from salt corrosion and heat. No existing paint could survive the temperatures seen in these new powerplants.
The hotter temperature in jet engines also cooked grease in throttle controls causing cables to stick and seize. In 1966, Teleflex Inc., a control manufacturer in North Wales, PA, patented a heat-curable, aqueous binder combining phosphoric acid with 5-6 wt. % chromic acid (50 to 60,000 ppm Cr+6). Mixing graphite into this binder created a slurry, which, when sprayed and baked, produced a coating that lubricated up to 1200oF (650oC).
Adding aluminum powder to the binder proved even more useful. The resulting Al-filled slurry, SermeTel™ W, was stable for a year. It sprayed like paint, yet, when baked at 600oF, cured to a tough, adherent aluminum-ceramic film that limited oxidation and salt corrosion of steels up to 1000oF [Fig 1]. The aluminum-ceramic was introduced on compressor cases of the J-79 engine, and, by 1965, Pratt, Detroit Diesel Allison (now Rolls-Royce), and GE were using it to protect steel shafts and cases in their turbines.
An Unexpected Capability
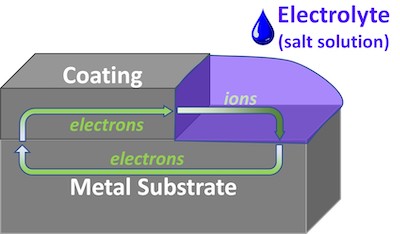 FIGURE 2: When damaged, a sacrificial metallic coating corrodes in place of the substrate, sacrificing electrons to preserve exposed metal.It was soon discovered that the aluminum-chromate/phosphate coating would become electrically conductive when it was baked for 90 minutes at 1050oF (566oC) or when lightly blasted with fine grit or beads. The resulting conductive coating was galvanically sacrificial to steel.
FIGURE 2: When damaged, a sacrificial metallic coating corrodes in place of the substrate, sacrificing electrons to preserve exposed metal.It was soon discovered that the aluminum-chromate/phosphate coating would become electrically conductive when it was baked for 90 minutes at 1050oF (566oC) or when lightly blasted with fine grit or beads. The resulting conductive coating was galvanically sacrificial to steel.
A sacrificial metallic coating is one that is more galvanically active than the substrate. If the coating layer is damaged so that the substrate is exposed, the coating corrodes in place of the substrate [Fig 2]. Galvanizing – coating iron or steel with a layer of zinc – is one commercial example of a sacrificial coating.
The capability of the conductive aluminum ceramic was striking. The coating provided galvanic protection up to 1000oF (540oC) and was impervious to water, oil, hydraulic fluids, and jet fuel. These and other properties [Table 1] made the coating ideal for turbine components.
TABLE 1: Capabilities of Sacrificial Aluminum-Chromate/Phosphate Primer
|
Abrasion Resistance |
Loses < 4 L of sand per micron of coating when tested with falling sand per ASTM D968. |
|
Oxidation Resistance |
No blisters and < 5% change in weight after 250 hrs. at 870°C (1600oF). |
|
Cyclic Oxidation-Corr Resis. |
No change through 6 cycles - 16 hrs. @ 540°C followed by 32 hr. in 5% neutral salt spray. |
|
Thermal Shock Resistance |
Coating intact after quench in running water following 4 hr. at 635°C. |
|
Sacrificial Corrosion Resistance |
No base metal attack through 1000 hrs. continuous exposure to 5% salt spray when sacrificial coating is scribed. |
|
Sacrificial Corrosion Protection |
Bare area 9.5mm diameter in the center of a sacrificial coating shows no red rust throughout 1000 hours in 5% neutral salt fog. |
|
Impact Resistance |
Remains bonded upon 72 inch-pound reverse impact with 16mm steel ball. No rust after 1000 hrs. in 5% salt fog following impact. |
|
Adhesion |
Does not loosen or flake when panel is bent 90 degrees around a mandrel 8 times in diameter than the panel thickness. |
|
Heat Resistance |
Does not crack or blister after heating for 23 hr. at 370°C ±15 (698oF), followed by 4 hr. at 580°C ±15 (1076oF). |
|
Hot Water Resistance |
No checking, blistering, or leaching thru 10 min. in boiling water. After 3 hr. rest, boiled coating does not flake in Adhesion test. |
|
Fuel Resistance |
Twenty-four hours after a 4 hr. RT soak in ASTM Reference Fuel B, coating does not flake when bent in Adhesion test. |
|
Hot Oil Resistance |
Thru 8 hours immersed in di-ester oil at 204°F ±15, coating shows no peeling or blistering and only slight softening, if any. |
Sources:
- MIL-C-81751B – COATING, METALLIC-CERAMIC, 17 January 1972
- PWA 595 – ALUMINUM COATING, first issued 1964-05-15
 FIGURE 3: A sealed, aerodynamically smooth sacrificial aluminum-ceramic coating on compressor airfoils for an industrial gas turbine. Airfoils coated with BOR1993, Courtesy of Blastech Overhaul & Repair Corp.As sacrificial aluminum-ceramic proliferated, the US Navy noted aggressive corrosion on some coated components in engines on carrier-based aircraft. Planes were exposed not only to salt but also to sulfur dioxide (SO2) in the exhaust from boilers and engines burning bunker fuels to power the warships. The SO2lowered the pH of moisture condensing in the engines to as little as 3.0, dramatically accelerating corrosion.
FIGURE 3: A sealed, aerodynamically smooth sacrificial aluminum-ceramic coating on compressor airfoils for an industrial gas turbine. Airfoils coated with BOR1993, Courtesy of Blastech Overhaul & Repair Corp.As sacrificial aluminum-ceramic proliferated, the US Navy noted aggressive corrosion on some coated components in engines on carrier-based aircraft. Planes were exposed not only to salt but also to sulfur dioxide (SO2) in the exhaust from boilers and engines burning bunker fuels to power the warships. The SO2lowered the pH of moisture condensing in the engines to as little as 3.0, dramatically accelerating corrosion.
Teleflex had just developed a chromate/phosphate sealer for SermeTel W. Adding this topcoat over the conductive Al-ceramic significantly extended the life of shipboard components. The Cr+6 topcoat sealed porosity in the burnished sacrificial primer while chemically passivating exposed aluminum.
Soon Teleflex’s Sermatech Division perfected a way to polish SermeTel W to make the sacrificial coating aerodynamically smooth. Further development brought a sealed smooth coating, SermeTel 5380DP. Applying 5380DP to blades and vanes increased compressor efficiency and reduced fuel consumption by as much as 2%. Coatings like this remain the standard for compressor airfoils in gas turbines [Fig 3].
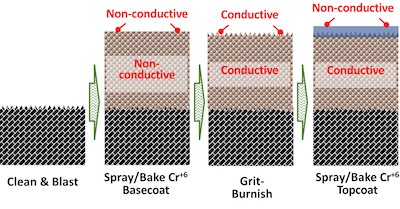 FIGURE 4: Process for depositing sealed, sacrificial aluminum-chromate/phosphate coating systems used in gas turbine engines today.In 1982, Coatings For Industry, Inc. (CFI) patented an aluminum-ceramic, Alseal™ 500, which cured at lower temperatures, enabling the use of Al-ceramic coating systems on aluminum & HSLA steel alloys.
FIGURE 4: Process for depositing sealed, sacrificial aluminum-chromate/phosphate coating systems used in gas turbine engines today.In 1982, Coatings For Industry, Inc. (CFI) patented an aluminum-ceramic, Alseal™ 500, which cured at lower temperatures, enabling the use of Al-ceramic coating systems on aluminum & HSLA steel alloys.
Consequently, since the mid-1980s, nearly every turbine manufacturer has used sacrificial aluminum-chromate/phosphate coating systems in their engines. The majority of these coatings utilize an electrically conductive (sacrificial) aluminum-ceramic primer, and chromate/phosphate topcoat applied as in Fig 4.
A Hidden Menace
But trouble lurked in the chromate/phosphates – Cr+6. All utilized binders like that originally patented by Teleflex, one that combines chromic acid (chromium trioxide) and phosphoric acid. Even when these coatings were introduced, it was known to treat chromic acid with care. OSHA had set Permissible Exposure Limits (PEL) requiring workers to breathe air containing no more than 0.1 mg Cr+6/cubic meter (m3). But concerns about hexavalent chromium (Cr+6) grew rapidly through the 1980s.
Soluble chromium exists primarily as trivalent [Cr+3] and hexavalent [Cr+6] ions. The reduced trivalent form is much less toxic and far more insoluble. In fact, trivalent chromium is an essential nutrient required by the human body. But, the hexavalent form of chromium [Cr+6] is very soluble, allowing it to pass through cell walls in mammals, making it a health risk. In 1987, the International Agency for Research on Cancer (IARC) concluded that Cr+6 raised the risk of lung cancer in workers exposed to acid fumes and chromate dusts.
The threat of Cr+6 broke into the public consciousness in 2000 with the release of the movie “Erin Brockovich.” Late in the ’90s, Cr+6 in the water supply had been identified as the cause of high cancer rates in Hinckley, CA. The movie dramatized the fight to bring the company responsible for the contamination to justice.
And more bad news followed about Cr+6. A study published in 2004 estimated that exposure at 0.1 mg Cr+6/m3, as then allowed under OSHA rules, permitted an excess risk of lung cancer death of more than 1 in 10 among more than ~1 million US workers exposed to that level of Cr+6 annually.
Acknowledging the risks of Cr+6, in 2006, OSHA lowered the PEL for Cr+6 to a mere 5 micrograms/m3 - a 20-fold reduction in the 8-hr. limit per employee! And the European Chemicals Agency (ECHA) classified chromium trioxide as a “substance of very high concern” and, in 2017, restricted its use in the EU. Under ECHA’s REACh regulations, users must now be authorized (for a substantial fee) to import any materials containing > 1000 ppm of Cr+6. (The original sacrificial chromate/phosphate primers contain as much as 30,000 ppm of Cr+6; topcoats as much as 55,000 ppm.)
The Cr-Free Quest Begins Simply
As early as 1990, suppliers and some users of chromate/phosphate coatings began developing Cr-free alternatives. Initial efforts simply eliminated chromic acid from the binder. Eventually, Sermatech International (now Praxair Surface Technologies) and others would patent more than a dozen acid-phosphate binders containing little or no hexavalent chromium. The first was issued in 1993 to Solar Turbines. That binder contained vanadium pentoxide (V2O5) instead of Cr+6 to stabilize Al in the slurry. Unfortunately, V2O5, though not carcinogenic, is poisonous.
While chemistries disclosed in these patents differ in detail, all describe a Cr-Free two-part aluminum-phosphate slurry that must be mixed before use. That’s because, without Cr+6, the aluminum began to react with the phosphate binder within a few minutes or, at most, hours.
The absence of Cr+6 also meant Cr-free acid-phosphate coatings reacted with blasted carbon steel unless it was first oxidized. For these reasons, by the turn of the century, the industry was no closer to a commercially viable Cr-Free alternative to the original SermeTel-type primers and topcoats than it had been a decade earlier.
Back to Square One
In 2001, CFI abandoned Cr-Free acidic phosphate (pH < 3) binder as the route to an alternative to Al-chromate/phosphates. Instead, CFI developed a heat-curable aluminum-filled slurry using a basic (pH > 7) inorganic aqueous silicate binder.
Aqueous silicate “water-glass” binders have been known for more than 150 years. They’ve long been used in inorganic zinc-rich paints. CFI’s single-component aluminum-silicate slurry has a one-year shelf-life. It is unreactive with steels, and when cured, it can be made electrically conductive and galvanically sacrificial by the same means the industry uses for Al-chromate/phosphates.
In 2003, CFI filed for a patent on its innovative slurry, which was eventually granted in 2017. In 2015, Praxair Surface Technologies received a patent on its own aluminum-silicate primer.
An Attempted Preemptive Strike
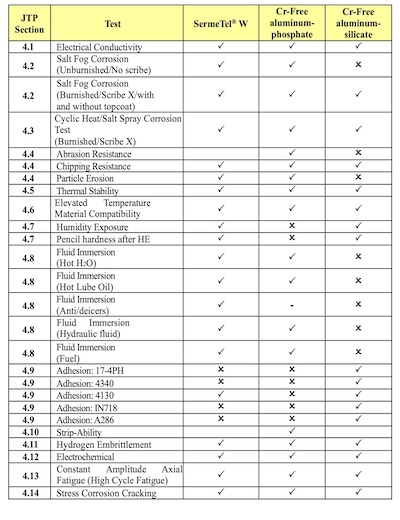 TABLE 2: Summary of results PWEG Tests of Sacrificial Aluminum-Ceramic PrimersAnticipating OSHA’s plan to dramatically lower the PEL for Cr+6 in 2006, in 2005, the US Dept. of Defense established The Propulsion Environmental Working Group (PEWG) to spur “green” innovation for turbine engines. PEWG marshaled “Industry and government leaders” to build “The Ultimate Green Engine.” This was defined as an engine:
TABLE 2: Summary of results PWEG Tests of Sacrificial Aluminum-Ceramic PrimersAnticipating OSHA’s plan to dramatically lower the PEL for Cr+6 in 2006, in 2005, the US Dept. of Defense established The Propulsion Environmental Working Group (PEWG) to spur “green” innovation for turbine engines. PEWG marshaled “Industry and government leaders” to build “The Ultimate Green Engine.” This was defined as an engine:
- Made with parts that lasted their designed lifetime,
- Containing no toxic materials,
- Built, maintained, and reworked without using or releasing any hazardous materials, with
- Valuable components and/or materials recoverable at life’s end.
PEWG selected SermeTel W to represent all existing sacrificial Cr+6 coating systems, and using existing OEM specifications for Cr+6 slurries as its guide, PEWG compared the performance of a Cr-Free sacrificial acid-phosphate system from Sermatech and the Alseal Al-silicate with a silicate-base topcoat to that of SermeTel W.
PEWG’s testing revealed no Cr-free alternative that matched the performance of the Cr +6 ‑Al-ceramic in every test [Table 2]. Consequently, PEWG chose to recommend the military use a “low Cr+6” Al-chromate/phosphate, one that still contained more than 1000 ppm Cr+6.
Discovering Synergy Between Cr-Free Materials
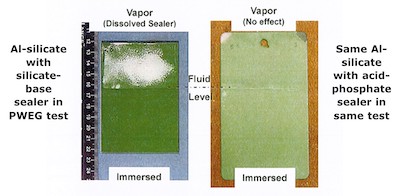 FIGURE 5: Steel panels coated with sacrificial Al-silicate primer and Cr-Free topcoats after partial immersion in deionized water for 24 hrs. at 120oC (250oC) in an open container. A Cr-Free silicate topcoat (left) dissolved in hot water in PWEG test. A topcoat using an acid-phosphate binder (right) did not. Source: ASTM GT2013-94465CFI’s Alseal aluminum-silicate primer was inherently stable in hot water, but PEWG tests showed that CFI’s silicate-based topcoat was not. This led CFI to try a topcoat made with a Cr-Free acid-phosphate binder over its aluminum silicate primer. That combination proved to be stable in hot water [Fig 5]. At last, this combination of an Al-silicate primer and acid-phosphate topcoat seemed to be the long-sought-after Cr-Free alternative to sacrificial Cr+6 aluminum-ceramic coating systems.
FIGURE 5: Steel panels coated with sacrificial Al-silicate primer and Cr-Free topcoats after partial immersion in deionized water for 24 hrs. at 120oC (250oC) in an open container. A Cr-Free silicate topcoat (left) dissolved in hot water in PWEG test. A topcoat using an acid-phosphate binder (right) did not. Source: ASTM GT2013-94465CFI’s Alseal aluminum-silicate primer was inherently stable in hot water, but PEWG tests showed that CFI’s silicate-based topcoat was not. This led CFI to try a topcoat made with a Cr-Free acid-phosphate binder over its aluminum silicate primer. That combination proved to be stable in hot water [Fig 5]. At last, this combination of an Al-silicate primer and acid-phosphate topcoat seemed to be the long-sought-after Cr-Free alternative to sacrificial Cr+6 aluminum-ceramic coating systems.
Fall Back (Again)!
But when CFI approached a power generation turbine OEM with its new Cr-Free system, it faced a new hurdle. That OEM had recently tested another Cr-Free coating in a turbine operating with wet compression. Wet compression injects water droplets into the inlet of a turbine to increase power by as much as 20%. Though the water eventually evaporates in the later stages of the compressor, blades and vanes in the first rows run wet all time.
 FIGURE 6: Front and back of 4130 steel panel coated with Alseal Cr-Free system (sacrificial Al-silicate primer plus acid-phosphate sealer) deposited in the “conventional” manner (FIGURE 4) after 100 hrs. sealed in a beaker partly filled with deionized water at 80oC (176oF).Though the Cr-Free coating in the OEM’s field trial had passed every conventional lab test, it blistered in service. The failure prompted the OEM to create a new screening test emulating wet compression. Coated panels were placed in a beaker partly filled with deionized water, sealed with plastic film, and held for at least 100 hr. at 80oC (176oF). CFI’s Alseal “improved” Cr-Free system utilizing an acid-phosphate topcoat over a sacrificial Al-silicate primer blistered in the test [FIGURE 6].
FIGURE 6: Front and back of 4130 steel panel coated with Alseal Cr-Free system (sacrificial Al-silicate primer plus acid-phosphate sealer) deposited in the “conventional” manner (FIGURE 4) after 100 hrs. sealed in a beaker partly filled with deionized water at 80oC (176oF).Though the Cr-Free coating in the OEM’s field trial had passed every conventional lab test, it blistered in service. The failure prompted the OEM to create a new screening test emulating wet compression. Coated panels were placed in a beaker partly filled with deionized water, sealed with plastic film, and held for at least 100 hr. at 80oC (176oF). CFI’s Alseal “improved” Cr-Free system utilizing an acid-phosphate topcoat over a sacrificial Al-silicate primer blistered in the test [FIGURE 6].
New Cr-Free Slurries Combined in A New Way
CFI returned to its development armed with this new screening test and with a new question. What if the key to an effective Cr-Free coating is not only found in new chrome-free slurries but also in combining these Cr-Free materials in new ways.?
To date, all sacrificial Cr-Free aluminum-ceramic systems have been built just like analogous Cr+6 systems; apply and cure a Cr-Free aluminum-filled primer, burnish that primer until it’s electrically conductive, and then seal the burnished primer with a Cr-Free topcoat as shown in Fig 4.
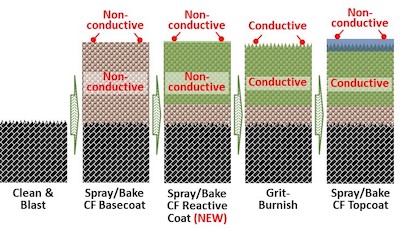 FIGURE 7: Novel way of depositing a more stable sacrificial Cr-Free Al-Ceramic System: Alseal 5KGT and 5KST. (Patent applied for)This new question led to a breakthrough - a multi-layer system in which the Cr-free aluminum-silicate basecoat is applied and cured and then treated with a Cr-Free acid phosphate solution before being burnished and sealed. This new construction scheme is shown in Fig 7. When CFI assembled its Cr-Free slurries in this way, the resulting coating system proved stable in hot water and water vapor for not merely 100 hrs. but for 1000 hours. [Fig 8]
FIGURE 7: Novel way of depositing a more stable sacrificial Cr-Free Al-Ceramic System: Alseal 5KGT and 5KST. (Patent applied for)This new question led to a breakthrough - a multi-layer system in which the Cr-free aluminum-silicate basecoat is applied and cured and then treated with a Cr-Free acid phosphate solution before being burnished and sealed. This new construction scheme is shown in Fig 7. When CFI assembled its Cr-Free slurries in this way, the resulting coating system proved stable in hot water and water vapor for not merely 100 hrs. but for 1000 hours. [Fig 8]
Coatings For Industry has designated its new multi-layered Cr-Free sacrificial aluminum-ceramic coating systems Alseal 5KGTand 5KST. As Table 3 shows, the performance of Alseal 5KST & 5KGT sealed, sacrificial coating systems is comparable to that of analogous multi-layer Cr+6 coating systems. Consequently, Alseal 5KGT and 5KST are now being evaluated by turbine engine OEMs. Alseal 5KGT has passed rigorous testing as a candidate for use on a flight turbine for the US Navy. Results of those trials were presented at NASF SUR/FIN 2022 in Rosemont, IL, this June. And in 2021, a major OEM of power generation turbines began field trials of Alseal 5KST on airfoils in select turbines.
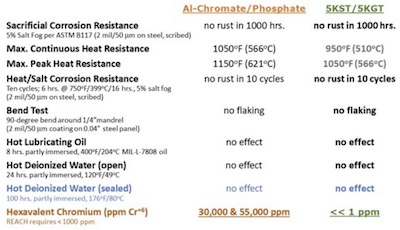 TABLE 3: New Alseal Cr-Free Coating Systems compare favorably with that of Cr+6 Systems currently in use.
TABLE 3: New Alseal Cr-Free Coating Systems compare favorably with that of Cr+6 Systems currently in use.
Conclusion
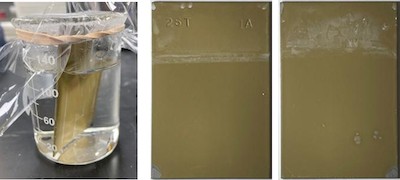 FIGURE 8: Front and back of steel panel coated with Alseal 5KST Cr-Free system deposited in the novel manner shown in FIGURE 7 after 1000 hrs. sealed in a beaker partly fill deionized water at 80oC (176oF).The history of the development of Alseal 5KGT/5KST Cr-Free coating systems demonstrates that the path to safe replacements for environmentally hazardous materials can be a long and tortuous one. It invariably includes underestimation of the task (something that has plagued innovators in this case). It can require as much unlearning of old technology as the development of new. And above all, it requires persistence, persistence, and (lastly) persistence. Coatings For Industry is confident that Cr-Free Alseal 5KST & 5KGT systems described above will be useful Cr-Free alternatives to chromate/ phosphate slurries. However, we are also sure that further innovation will be needed and continue to labor to that end.
FIGURE 8: Front and back of steel panel coated with Alseal 5KST Cr-Free system deposited in the novel manner shown in FIGURE 7 after 1000 hrs. sealed in a beaker partly fill deionized water at 80oC (176oF).The history of the development of Alseal 5KGT/5KST Cr-Free coating systems demonstrates that the path to safe replacements for environmentally hazardous materials can be a long and tortuous one. It invariably includes underestimation of the task (something that has plagued innovators in this case). It can require as much unlearning of old technology as the development of new. And above all, it requires persistence, persistence, and (lastly) persistence. Coatings For Industry is confident that Cr-Free Alseal 5KST & 5KGT systems described above will be useful Cr-Free alternatives to chromate/ phosphate slurries. However, we are also sure that further innovation will be needed and continue to labor to that end.
Bruce McMordie is the Director of Alseal Operations at Coatings For Industry, Inc. in Souderton, PA. Visit www.cficoatings.com. The author has over 40 years’ experience developing and implementing coating systems to protect components in turbine and industrial equipment. He has spent more than 30 years with Sermatech International (now Praxair Surface Technologies) and 10 years with Coatings For Industry and holds 18 coating related patents.
Acknowledgments: Many thanks to Bradford Wiley of Rolls-Royce, Indianapolis, for championing Cr-Free alternatives to Cr+6 materials and for inviting Coatings For Industry to participate in NASF SUR/FIN 2022.
Notes
- SermeTel is a Trademark of Praxair Surface Technologies, Indianapolis, IN.
- Alseal is a Trademark of Coatings For Industry, Inc., Souderton, PA.
- A patent has been applied for the sacrificial aluminum-ceramic coating systems made with Cr-
- Free materials are described in this article.
References
- Allen, US Patent 3,248,251, INORGANIC COATING AND BONDING COMPOSITION, issued April 26, 1966.
- Das & S. Mishra, Hexavalent Chromium (VI): Environmental Pollutant and Health Hazard, Nat. Inst. Of Tech., Orissa (INDIA), Journal of Environmental Research and Development, Vol. 2 No. 3, Jan-March 2008, 386-392.
- Mosser, M. F., 1989, Compressor Disk Corrosion Problems and. Solutions, SAE Technical Paper 890916.
- Mosser M. F., 1988, An Improved Coating Process for Steel Compressor Components – SermeTel Process 5380DP, SAE 880879.
- De Flora S, Threshold Mechanisms and site specificity in Cr(VI) carcinogenesis, Carcinogenesis, 21(4), 533-541, (2000).
- Mancuso, T.F. (1997) Chromium as an industrial carcinogen: Part I, Am. Journal of Industrial Medicine, 31, 129-139.
- Gibb, H.J. et. al. (2000), Lung cancer among workers in chromium chemical production. Amer. Journal of Industrial Medicine, 38, 115-126.
- Luippold, R.S. et. al. (2003) Lung cancer among chromate production workers, Occupational and Environmental Medicine, 60, 451-457.
- Zhang et. al., Chronic Occupational Exposure To Hexavalent Chromium Causes DNA Damage In Electroplating Workers, BMC Public Health 2011, 11:224.
- US Dept. of Labor, Occupation Health and Safety Admin., 1910.1026 - Chromium (VI) https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1026
- European Chemicals Agency (ECHA), Substances Restricted under REACH, https://echa.europa.eu/substances-restricted-under-reach/-/dislist/details/0b0236e1807e2bc1
- Stetson et al., US Patent 5,242, 488, COATING COMPOSITION AND METHOD OF FORMING, Sept. 7, 1993.
- Stetson et al., US Patent 5,279,649, COATING COMPOSITION . . ., Jan. 18, 1994
- Mosser et. al US 5,478,413,. 26-DEC-1995; Mosser et. al. US 5,652,064, 29-JUL-1997, Mosser et. al., US 5,803,990, 08-SEP-1998; Myers et. al., US 5,968,240, 19-OCT-1999, Eddinger et. al. US 6,074,464, 13-JUN-2000; Mosser, US 6,150,033, 21-NOV-2000, and other patents.
- Mosser et al., US Pat. 7, 993, 438, HIGH TEMPERATURE RESISTANT COATING COMPOSITIONS, Aug. 9, 2011.
- Klotz et al., US Pat. 9,739,169, FORMATION OF CORROSION-RESISTANT COATING, August 22, 2017.
- Belov et al., US Pat. 9,017,464, CHROMIUM-FREE SILICATE-BASED CERAMIC COMPOSITION, April 28, 2015.
- McMordie et. al., ELIMINATING CARCINOGENS IN COMPRESSOR COATINGS, ASME GT2013-94465, Proceedings of the International Gas Turbine Engineering Conference, June 3-7, 2013, San Antonio, TX, USA.



































