Years ago, hard chrome shops knew the value of keeping their baths clean and free of impurities.
 Eric Svenson Sr.Their simple approach was to dilute and replace a portion of their baths whenever needed to reduce their impurity levels. They could tell by simple observation of the mist color when this was needed. This was before convenient laboratory services were available that could pinpoint exact impurity levels. All of that changed around 1970 with the EPA and the high cost of hazardous disposal. Plating shops could no longer afford the cost of hauling their waste away.
Eric Svenson Sr.Their simple approach was to dilute and replace a portion of their baths whenever needed to reduce their impurity levels. They could tell by simple observation of the mist color when this was needed. This was before convenient laboratory services were available that could pinpoint exact impurity levels. All of that changed around 1970 with the EPA and the high cost of hazardous disposal. Plating shops could no longer afford the cost of hauling their waste away.
Busy work schedules caused us to overlook the benefits of keeping the chrome bath clean. Over the years, impurities like trivalent, iron, and copper gradually built up in the baths. This resulted in much higher plating costs and a reduction in deposit quality. Most plating shops have since accepted this as the norm and the way things are supposed to be.
Several forward-looking shops, however, purchased bath purification equipment. But is this equipment really practical, and is it cost-effective? The simple answer is no, as most have not lived up to their claims and the operational costs are very high.
Now is the time to revisit this subject as disposal costs are now much lower. Here we will look at the pros and cons of various purification techniques and present a detailed cost study of these.
The Final Result Is Shocking
This project was started when a Venezuelan company built a new plant to manufacture and chrome plate large quantities of positive displacement pump rotors. The company was concerned about maintaining their baths in a pure state to keep their costs down and preserve their deposit quality.
We started this project by investigating the latest technology in both purification methods. The two most prominent ones seemed to be:
- Ion Exchange
- Membrane Electrolysis
But, we had no firm ideas of the cost involved with buying, operating, and maintaining these systems. Several purification equipment manufacturers were interviewed, and from this, we were able to come to some conclusions and provide a recommendation and cost options for the company and the industry.
It turns out that the “old school” dilution and remaking is by far the best approach. Today, this is even less expensive than keeping an old impure bath.
Why? It’s because the operational costs involved with plating in a bath loaded with trivalent and metals are so high combined with today’s much lower disposal costs.
Objective
Our objective is to review various bath purification technologies and their related costs and to learn the actual cost of operating baths with high levels of impurities. Maintaining chrome plating baths at low impurity levels is critical if high-quality deposits and an efficient operation are desired. Keeping the plating bath pure also reduces defects such as pitting and significantly lowers the electrical energy requirements.
Some of the problems that high impurity levels cause include:
Quality-Related
- Deposit pitting
- Burning and roughness
- Dull deposits
- Trees and nodules
- Poor coverage (throw) and skip-plating
- Blistering and peeling
- Deposit stress and brittleness
- Softer deposits
- Reduced wear resistance
- Macro-cracking
- Poor corrosion resistance
- Limiting the available rectifier current
Business Related
- Slower plating speeds
- Excessive stripping and rework
- Lower production rates
- Higher electrical costs
Typical Impurities
 Any ion not intentionally included in the bath is considered an impurity, but the most common ones are chloride, trivalent, iron, copper and other heavy metals. The goal is to maintain chrome plating baths in as pure a state as possible. Any ion not intentionally included in the bath is considered an impurity, but the most common ones are chloride, trivalent, iron, copper, and other heavy metals. The primary impurities include:
Any ion not intentionally included in the bath is considered an impurity, but the most common ones are chloride, trivalent, iron, copper and other heavy metals. The goal is to maintain chrome plating baths in as pure a state as possible. Any ion not intentionally included in the bath is considered an impurity, but the most common ones are chloride, trivalent, iron, copper, and other heavy metals. The primary impurities include:
Trivalent and Chloride
The ideal trivalent level is 1% of the chromic acid in the bath, or about 0.3 oz/gal (2.25 g/l) for most baths. Anything above 1% is considered an impurity. Levels of 4%-5% or more are considered extreme. Trivalent complexes the chromic acid by a factor of about 2.4 oz/gal for each percent present. This upsets the proper sulfate ratio.
Example: A 30 oz/gal bath with 5% trivalent has a usable chrome level of only 18 oz/gal, so the effective ratio is reduced from 100:1 to only 60:1. Excess trivalent has about six times the negative effect that iron does. High trivalent levels also reduce the tolerance to other impurities. Excess trivalent is formed whenever the anode area is smaller than the cathode as in ID plating, with poor anode electrical contact when using heavily scaled (or yellow) lead alloy anodes, when using steel anodes, with excessive reverse etching, and whenever metals like steel or copper are dissolved, or where organics such as oils are introduced into the bath.
The ideal chloride (Cl) level is below 20 ppm, with 50 ppm or more considered excessive. Chloride acts as an extremely powerful catalyst that upsets the sulfate ratio. A level of 100 ppm has the effect of an additional 0.10 oz/gal of sulfate. The most common chloride source is drag-in from a hydrochloric acid stripper. Other sources can include the water supply, breakdown of PVC tank linings and piping, and impure chromic acid. DI water systems are recommended to eliminate excess chloride in the water supply. Precipitation of chloride with silver compounds, while effective, is cost prohibitive.
Fortunately, both trivalent and chloride are fairly easy and inexpensive to remove by simple dummying. Dummying converts the trivalent back into chromic acid and drives off chlorides at the anode surface.
CR-r Reducer
This helps to remove these impurities when dummying. Use as large an anode area as possible (a 30:1 ratio is best but hard to achieve), along with a high bath temperature and current. Under these conditions, the removal rate is 2% trivalent and 50 ppm of chloride per 24 hours; double these times for a 15:1 ratio. Longer times will be needed for lower ratios, temperatures, or current settings. Also, lower impurity levels are reduced slower than higher levels are. So, while reducing the trivalent from 5% to 3% may only take 24 hours, going from 3% to 1% will take longer.
Shops with a constant trivalent (or chloride) problem should continuously operate a separate dummying tank. Porous pots are also effective for use in smaller tanks where space is available. Regardless of the method, the addition of 1%-2% of CR-3 Reducer helps greatly in removing both trivalent and chloride.
Iron, Copper, and other Heavy Metals
The total heavy metals level (iron, copper, nickel, etc.) should be kept below 5 g/l (combined) for most operations. In critical applications, these should be below 1-2 g/l. Combined metal impurity levels of 8 g/l, or more, are considered extreme. Contrary to older beliefs, these metals do not plate out, so they only continue to build up in the bath. And as these metals are introduced into the bath, they also increase the trivalent level by an equal amount. Like trivalent, these metals combine with the chromic acid present to form an ionized complex that has the effect of lowering the effective sulfate ratio.
Iron contamination primarily comes from reverse etching in the plating bath and from cathodic attacks in unplated areas. Shops that use steel anodes will have a very high iron level. The tendency for deposit pitting is reduced by almost 90% when lowering the iron from 8 g/l to 1 g/l. Iron levels higher than 10 g/l greatly reduce adhesion and cause deposit brittleness.
Copper is even more of a concern as its negative effect is stronger than iron. Copper impurities typically come from an attack on the fixtures, bussing, and any copper-based parts that get plated.
Unfortunately, an inexpensive method of removing large quantities of iron or copper impurities doesn’t exist. The use of Ion Exchange and Membrane Electrolysis is discussed below, but as you will see, neither is a good option. Porous pots may be effective for use in smaller tanks where space is available. An addition of 1%-2% of CR-3 Reducer helps greatly when using porous pots.
Dura-76
This can be used to chelate these metals if the impurity level is not too high. This seems to work well if no other remedy, such as removal, is used.
Operational Costs
This shows the costs involved in operating a bath that’s high in impurities versus one that has been purified. This is based on a typical shop with one (1) 2,000-gallon bath operating on a single-shift basis. After one year of operation, the Total Impurity Index level was 11. They decided to maintain it between 4.8 and 7.2; i.e., whenever it reached 7.2, they would treat the bath to lower it to 4.8.
Cost To Operate An Impure Bath
- Higher electrical costs: $16,907 (higher voltage requiring more AC)
- Slower plating speed: $116,805 (58% slower than a clean bath)
- Stripping and re-plating:$56,066 (rework rate x 2 for strip and re-plate)
- Total: $189,778
Costs To Purify With Ion Exchange
- Ion-X system amortization: $7,865
- Waste treatment system amortization: $7,150
- Regeneration chemicals: $2,949
- DI water used: $759
- Resin changes: $1,287
- Filter tubes: $286
- Regenerate sludge disposal: $67,457
- Electricity to operate: $164
- Maintenance and replacement parts: $715
- Operation and maintenance labor: $3,575
- Total: $92,207
Cost To Dilute and Replace Baths
- Chemicals for bath make-up: $25,767
- Bulk waste hauling: $20,300
- Total: $46,067
Most shops don’t fully realize the extra costs involved in keeping a bath that’s high in impurities. Yes, using ion exchange will save some money, but you need to purchase an ion-x system and a waste treatment system. The capital outlay for these could exceed $150,000. In addition, you will need extra labor to operate and maintain these systems, labor that could be used for plating parts and generating profits.
What’s really astonishing is the amount of money this small but busy shop was able to save by replacing a portion of their bath on a regular basis. This amounted to over $ 143,000 per year in extra profits. They were also able to plate much more work (due to the faster speeds) and therefore generate extra profits.
All you need to do is call a bulk licensed waste hauler to pump out a portion of your tank whenever the impurities are at their limit and then make up the extra bath. The volume and frequency are based on the impurity build-up rate and will vary depending on the operation. Part of what makes this work is today’s much lower waste hauling costs.
A spreadsheet program was developed that pinpoints these costs based on a shop’s particular situation. This data is available to anyone interested; simply call us for your specific numbers.
Dilution and Replacing Baths
Diluting and remaking chrome baths is the most practical and economical means of controlling impurities. In the “old days,” plating shops did this routinely as they knew the value of keeping their baths clean. This allowed them to plate faster and produce better quality work. The savings went straight to the bottom line; they were able to stay competitive and get more work out the door.
Periodic dilution is the best way to keep iron, copper, and other heavy metal impurities in check. If the trivalent or chloride is also high, it may make sense to dummy the bath first. Dummying first will lower these and may result in a smaller bath volume that needs to be remade, thusly saving even more expense.
Unfortunately, many chrome shops today live with the problems of high bath impurities and have come to accept slower speeds, poorer quality work, higher rejects, and much higher electrical costs. It doesn’t need to be this way, as the same technique is again usable due to the much lower bulk waste hauling costs.
Low Concentration Bath
When using this approach, it also makes sense to use a low-concentration bath. Several 20-ounce and 25-ounce baths are now available that reduce the make-up and use costs even further. Using a low concentration bath also:
- Lowers the chromic acid used.
- Slows the impurity build-up rate.
- Increases the plating speed.
- 50% less misting for better OSHA and EPA numbers.
Ion Exchange and Membrane Electrolysis
Data on these two purification methods are included so the reader has a better understanding of their operation. As indicated, Ion exchange, while workable, is not cost-effective. The short version of Membrane Electrolysis is that it is totally impractical and is fraught with operational problems.
Of the two methods, ion exchange is the most practical and has been in use since 1970. Two companies currently manufacture Ion-X systems for chrome purification. A special cation resin is used that is resistant to the oxidizing chromic acid. Impurity removal occurs on the ion exchange resin that’s made from tiny beads of long-chain hydrocarbons, such as polystyrene, which have sites attached that are loaded with H+ ions. As the bath passes through the resin column, it exchanges the H+ ion (harmless to the bath) for the impurities Fe or Cu ions. Once loaded, the resin is regenerated with sulfuric acid. This creates large quantities of wash-down liquid that needs to be waste treated. This wash-down contains sulfuric acid, the impurities removed, and chromic acid. The problem with Ion-X is the high cost of the equipment and waste treatment needed and the tremendous amount of hazardous sludge generated. Waste treatment and sludge disposal account for over 80% of the operational cost.
The Membrane Electrolysis method (electrodialysis), while great in theory, leaves a lot to be desired in terms of practicality and efficiency. The technology is not yet fully developed and may never be. The main problems are:
- The membrane maintenance requirements; cost, and labor are very high
- Maintenance of the catolyte (acid or alkaline)
- Removing, handling, and disposing of the impurities after removal.
Membrane Electrolysis will not reduce the impurities to low levels, and the removal rate is very slow. In general, membranes are not very efficient for Fe and Cu removal, but they are slightly more efficient for trivalent. These membranes are very delicate and are easily damaged during maintenance; their replacement amounts to about 65% of the cost of the system. While some companies still make this equipment, several have gone out of business in recent years.
Removal Rate Required
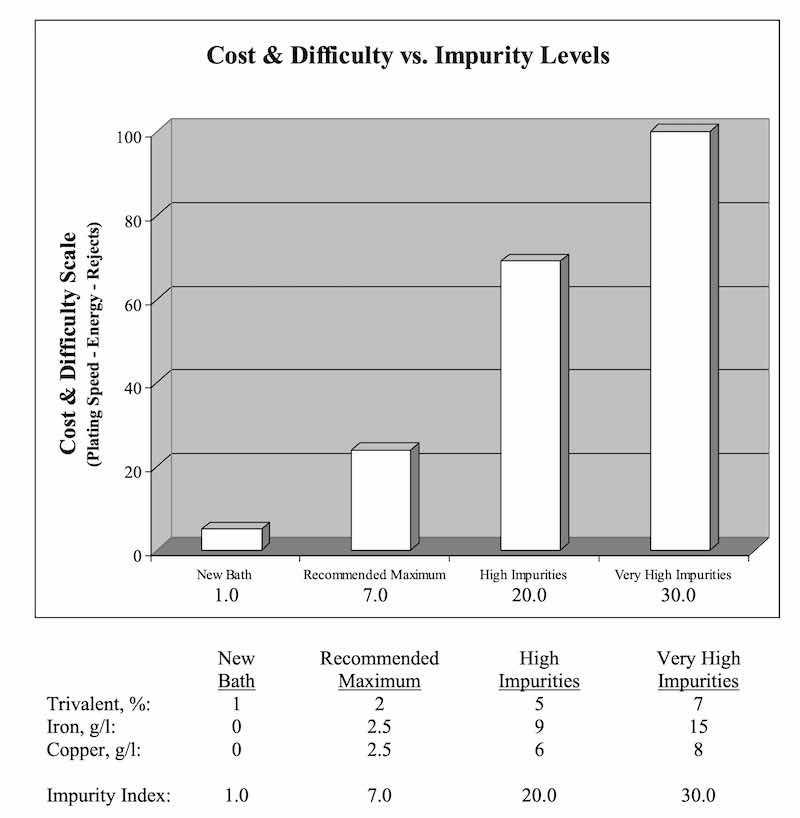 Our first concern is determining the rate the Total Impurity Index builds up in the bath(s), which is usually calculated on a monthly basis. This tells us the treatment frequency needed to maintain the impurities within an established range. The bath(s) are treated to the low point of the Index, then again whenever they reach the high. The highs and lows are typical +/- 20% of the ideal level.
Our first concern is determining the rate the Total Impurity Index builds up in the bath(s), which is usually calculated on a monthly basis. This tells us the treatment frequency needed to maintain the impurities within an established range. The bath(s) are treated to the low point of the Index, then again whenever they reach the high. The highs and lows are typical +/- 20% of the ideal level.
This build-up rate is easily determined by analyzing bath samples, say 90 days apart, and should be done during normal production periods. Baths that are heavily loaded with impurities may need a larger portion removed initially. Each treatment afterward would then be a much smaller volume and thusly less expensive.
Conclusion
There are tremendous operational savings available by using the Dilution and Replacing approach. You shouldn’t look at this as an added expanse but instead as an opportunity to generate extra profits.
Consider the following:
- Bulk waste hauling costs have been drastically reduced
- Assistance is available in locating a suitable low-cost bulk waste hauler
- The use of the newer Low Concentration baths will keep your chemical costs down
- Assistance is available to determine your Impurity Index level and treatment frequency
- Also available is a no-cost analysis of the savings this will provide for your operation.
Eric Svenson Sr. is CEO of Plating Resources, Inc. Visit http://www.plating.com



































