This article discusses the development of a new copper plating bath that can deposit high ductility copper metal directly on a wide range of aluminum alloy substrates.
 Richard DePoto and Doug DudaThe current process of record (POR) requires that a zincate or a double zincate pretreatment step be applied to the aluminum before the copper can be plated on to the aluminum substrate. The development of this newly designed copper plating bath eliminates the requirement for the costly and time-consuming zincate pretreatment steps, requires fewer processing steps, and is less costly. The process is particularly important for general metal finishing companies looking to plate aluminum parts for aerospace industry.
Richard DePoto and Doug DudaThe current process of record (POR) requires that a zincate or a double zincate pretreatment step be applied to the aluminum before the copper can be plated on to the aluminum substrate. The development of this newly designed copper plating bath eliminates the requirement for the costly and time-consuming zincate pretreatment steps, requires fewer processing steps, and is less costly. The process is particularly important for general metal finishing companies looking to plate aluminum parts for aerospace industry.
Introduction
There are numerous potential applications for lightweight aluminum to be used as a replacement for heavier and more costly metal structures. Applications for the expanded use of aluminum in electronics include bus bars, switch gears and terminal boards. In these applications, aluminum would replace copper. Additionally, in the automotive industry, although aluminum is commonly used, its use has been somewhat restricted due to the costly and complicated soldering techniques required to attach aluminum to other metal surfaces. If aluminum could be provided with a thin layer of well adherent copper, the utility of aluminum’s inherent light weight and strength properties could be dramatically expanded. The current Process of Record (POR) to achieve adhesion utilizes a zincate conversion layer that is costly, unreliable and wears away at the aluminum surface, causing undesirable dimensional variation. An answer lies in the direct copper metallization of aluminum.
The claims for direct copper metallization are numerous. The process is said to simplify and reduce the number of steps required to plate copper on aluminum substrates by eliminating the required zincate conversion steps. It addresses the adhesion of the copper to low potential substrates such as aluminum, steel and stainless steel. With this process, copper adhesion to the aluminum substrate can be successfully soldered with lead free solder without peeling or blistering. An alkaline cyanide-free copper can provide adhesion performance to pass ASTM-B-571 spec for thermal baking 240°C for 60 minutes. Finally, the improved process should result in minimal or no removal of aluminum metal in the pretreatment steps.
The Existing Zincate Process
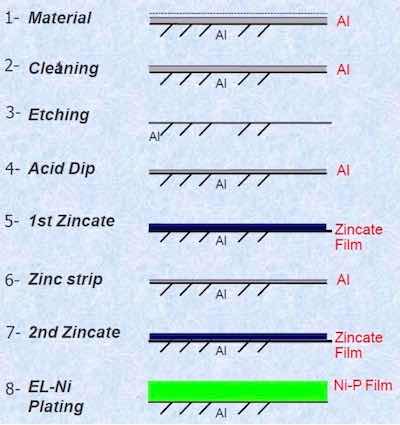 Figure 1: Zincate process steps.The Process of Record involving zincate is described in Fig. 1. Initially, the part surface to be plated contains dirt through prior handling, as well as a naturally-formed oxide film. The cleaning step removes the surface soils and enhances the wettability of the surface. Next, the oxide film is removed by etching. A subsequent acid dip reforms the oxide film, although it is thinner and more uniform. An initial rough zincate film is first applied, where aluminum is dissolved, and zinc ions are reduced and deposited. This coating is then stripped to yield another thin oxide film. The second zincate step produces a uniform zinc film with enhanced adhesion, compared with the first zincate. Finally, an electroless nickel deposit is applied; most of the zinc is dissolved and a strongly adherent Ni-P film is available for subsequent plating.
Figure 1: Zincate process steps.The Process of Record involving zincate is described in Fig. 1. Initially, the part surface to be plated contains dirt through prior handling, as well as a naturally-formed oxide film. The cleaning step removes the surface soils and enhances the wettability of the surface. Next, the oxide film is removed by etching. A subsequent acid dip reforms the oxide film, although it is thinner and more uniform. An initial rough zincate film is first applied, where aluminum is dissolved, and zinc ions are reduced and deposited. This coating is then stripped to yield another thin oxide film. The second zincate step produces a uniform zinc film with enhanced adhesion, compared with the first zincate. Finally, an electroless nickel deposit is applied; most of the zinc is dissolved and a strongly adherent Ni-P film is available for subsequent plating.
Alkaline cyanide-free copper plating
The alkaline cyanide-free copper plating process discussed here is based on pyrophosphate chemistry. In contrast to the zincate process, the process steps preceding the actual copper plating are (a) alkaline soak cleaning to remove surface soils and increase wettability, and (b) a 50% nitric acid dip to remove the naturally-occurring oxide film. From here, subsequent deposits, including tin, gold, nickel, etc., can be applied.
Copper pyrophosphate is the main raw material for the preparation of this copper plating bath. It reacts in the bath with potassium pyrophosphate, the complexing agent, to form pyrophosphate copper ligand ion:
Cu2P2O7 +3K4P2O7 ® 2K6[Cu(P2O7)2]
Converts ® 6K[Cu(P2O7)2]6+2 ® Cu+2
Cu+2 + 2e– ® Cu metal
The bath makeup and operating conditions are given in Table 1. The simple four-component system uses an EDTA-free biodegradable complexing agent which allows a near neutral pH operating range. Additives are replenished on the basis of ampere-hours. For rack plating, the optimum current density is 20 A/ft2 (Range = 10-40 A/ft2), with an anode-to-cathode ratio of 2:1. The barrel plating regimen operates at 5 A/ft2 (Range to 10 A/ft2), with an anode-to-cathode ratio of 1:1. Filtration is continuous.
Table 1- Copper pyrophosphate bath chemistry and operating conditions
| Parameter | Optimum | Range |
| Metal salt | 80 g/L | 40-100 g/L |
| Copper as m | 28 g/L | 20-34 g/L |
| Conductivity | 270 g/L | 240-300 g/L |
| Complexing | 50 mL/L | 40-60 mL/L |
| Brightener | 10 mL/L | 8-15 mL/L |
| Temperature | 149°F | 140-158°F |
| pH | 7.5 | 7.0-8.5 |
Live entry into the plating bath is essential. The copper content in the plating bath must be controlled at 20-34 g/L, as it has a significant effect on the cathodic polarization and the current density operating range. The result will be low brightness and smoothness. High copper content will decrease the cathode polarization and result in a rough plating coat (Fig. 2). High copper and high buildup of orthophosphate reduces the conductive ability, increases the competing immersion copper reaction and a potential for adhesion loss will increase. Bath agitation is very important for ion replenishment. We have worked on a special eductor design for this bath, but air agitation has been successfully used. Nonetheless, the process does require tighter bath control than acid or cyanide copper baths require.
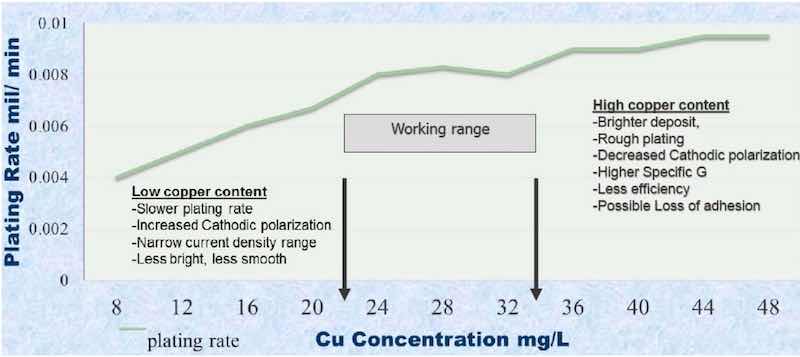 Figure 2: Plating rate vs. copper content.
Figure 2: Plating rate vs. copper content.
The operating current density has a wide range, but thickness control and uniformity are both important (Fig. 3). Proper control of pH is necessary (Fig. 4). A low pH precipitates the complexor, while high pH precipitates copper hydroxide (Cu(OH)2).
 Figure 3: Current density vs. deposit quality index
Figure 3: Current density vs. deposit quality index
 Figure 4: Plating rate vs bath pH.
Figure 4: Plating rate vs bath pH.
Compared to acid and cyanide copper processes, copper pyrophosphate baths are characterized by high stability. The deposit is a meticulous crystalline coating and has a better coverability than acid copper plating. The operating bath has a higher cathodic current efficiency than cyanide copper plating. It is an excellent bath for plating thick copper. Further, a thick coating can be obtained with low embrittlement due to the fact that no gas is generated from the electroplating process (high current efficiency).
Table 2 - Stress testing: SAC 305 Solder Shock Test
| Copper plate plate thickness (mils) 0.50 | Test result after SAC 305 255°C/10 sec Immersion test |
| 0.75 | No delamination |
| 1.0 | No delamination |
| 1.5 | No delamination |
| 1.75 | No delamination |
| 2.0 | No delamination |
| 2.5 | ~20% Failure at Al/Cu Interface |
| 3.0 | >25% Failure at Al/Cu Interface |
Properties and Testing
 Figure 5: Solder shock test results showing (a) no Al-Cu delamination with a Cu thickness of 1.0 mil and (b) failure and separation with a 2.5-mil Cu deposit (Ni over Cu).One of the primary criteria for use in electronic applications is solderability. The direct copper-plated aluminum must be able to undergo the thermal shock of soldering without loss of adhesion through heat stress. In this evaluation, we used a SAC 305 solder shock test, subjecting a test sample to immersion in SAC 305 solder for 10 sec at 255°C. SAC 305 is a lead-free alloy consisting of 96.5% tin, 3.0% silver and 0.5% copper. The test sample should exhibit no delamination after the procedure. Samples were prepared with copper thicknesses ranging from 0.5 to 3.0 mils. As shown in Table 2 and Fig. 4, no delamination was observed in the lower thickness samples, from 0.5 to 2.0 mils. However. at thicknesses above 2.0 mils, separation occurred at the aluminum-copper interface over 20% of the area of the part. The failure area increased with thickness. It is apparent that the solder shock test induces stress at the aluminum-copper interface, and that higher copper plating thicknesses increases the failure rate.
Figure 5: Solder shock test results showing (a) no Al-Cu delamination with a Cu thickness of 1.0 mil and (b) failure and separation with a 2.5-mil Cu deposit (Ni over Cu).One of the primary criteria for use in electronic applications is solderability. The direct copper-plated aluminum must be able to undergo the thermal shock of soldering without loss of adhesion through heat stress. In this evaluation, we used a SAC 305 solder shock test, subjecting a test sample to immersion in SAC 305 solder for 10 sec at 255°C. SAC 305 is a lead-free alloy consisting of 96.5% tin, 3.0% silver and 0.5% copper. The test sample should exhibit no delamination after the procedure. Samples were prepared with copper thicknesses ranging from 0.5 to 3.0 mils. As shown in Table 2 and Fig. 4, no delamination was observed in the lower thickness samples, from 0.5 to 2.0 mils. However. at thicknesses above 2.0 mils, separation occurred at the aluminum-copper interface over 20% of the area of the part. The failure area increased with thickness. It is apparent that the solder shock test induces stress at the aluminum-copper interface, and that higher copper plating thicknesses increases the failure rate.
A second thermal stress test was undertaken, involving a thermal bake at 240°C for 60 min, followed by an immediate quench in water, as per ASTM B-571. The same criterion applied for passing the test, i.e., no delamination. The results are shown in Table 3. Here, no delamination was observed with thicknesses in the 0.5 to 1.5 mil range. From 1.75 to 3.0 mils however, delamination failure occurred, increasing with thickness from ~5% to ~25% area failure. These results indicate that the thermal bake and quench stress test is more severe than the solder shock test, and that higher copper plating thicknesses show an increased failure rate. This is quite apparent in Fig. 6, where the results for copper plated Al 3003 are compared.
Table 3 - Stress test: ASTM B-571 Thermal Bake+Water Quench
|
Copper plate plate thickness (mils) 0.50 |
Test result after SAC 305 255°C/10 sec Immersion test |
| 0.75 | No delamination |
| 1.0 | No delamination |
| 1.5 | No delamination |
| 1.75 | ~5% Failure at Al/Cu Interface |
| 2.0 | ~10% Failure at Al/Cu Interface |
| 2.5 | ~20% Failure at Al/Cu Interface |
| 3.0 | >25% Failure at Al/Cu Interface |
There are a multitude of aluminum alloys, used in aerospace and electronic applications, with varying silicon content. Several of these alloys were thermal stress tested with the ASTM B-571
240°C/60 min bake/quench test. The results, shown in Table 4, indicate that the compatible alloys tested pass which contained less than 1% silicon.
Table 4: Stress test: ASTM B-571 Thermal Bake+Water Quench for a variety of silicon-containing aluminum alloys.
| Al alloy | Silicon content (%) | Results after plating test | Results after 240°C/60 min bake | ||
| 1008 | 0.40% | Pass | Pass | ||
| 2024 | 0.25% | Pass | Pass | ||
| 3003 | 0.60% | Pass | Pass | ||
| 4130 | 0.25% | Pass | Pass | ||
| 5052 | 0.25% | Pass | Pass | ||
| 6061 | 0.60% | Pass | Pass | ||
| 7050 | 0.12% | Pass | Pass | ||
| 4006 | 1.00% | Pass | 5-10% adhesion loss | ||
Pretreatment Cycles
In addition to aluminum alloy substrates, it was important to determine the proper pretreatment cycle for plating this pyrophosphate copper on other substrates, in particular, for zinc die-castings and stainless steel. Cycles were determined for both and are summarized in Table 5.
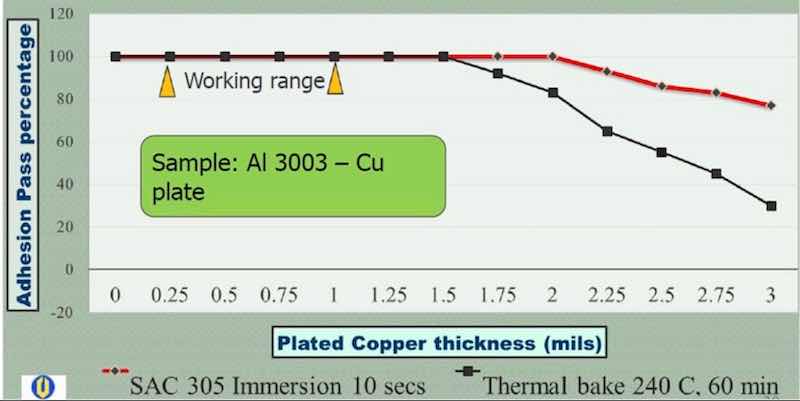 Figure 6: Comparison of thermal stress test results: Copper on Al 3003 alloy.
Figure 6: Comparison of thermal stress test results: Copper on Al 3003 alloy.
Table 5: Pretreatment cycles for pyrophosphate copper on important substrates.
| Substrate | Aluminum | Zinc Die-cast | Stainless Ste |
| Cleaner | Electrocleaner | Soak cleaner | Soak cleaner* |
| Rinse | DI Water | DI Water | DI Water |
| Acid / Clean | 50% HNO3 | 1% H2SO4 | Acid electrocleaner |
| Rinse | DI Water | DI Water | DI Water |
*Proprietary
Conclusions
- Successful direct metallization of aluminum with a thin neutral cyanide-free copper overplate can be achieved.
- Zincate adhesion layers can be eliminated for a variety of common aluminum alloys.
- There is a direct and causal relationship between the plated copper thickness and the resulting adhesion. Copper deposits of 1.0 mils or less can be successfully soldered and survive thermal baking at 240°C for one hour without adhesion loss.
- Opportunities exist to expand the use of aluminum to replace steel and copper in the aerospace and automotive industry.
- The use of neutral pH cyanide -free copper as a “super strike bath” seems apparent and should enhance common overplates such as silver, palladium, bright nickel.
The Way Forward
- We plan to evaluate combining a thin copper-pyrophosphate strike deposit with a high-elongation bright acid copper to enhance adhesion and manage expansion mismatch.
- We have begun to look at specialty aluminum micro-etches to increase mechanical anchoring and increase adhesion of copper to aluminum.
- Plans call for continued work with a copper pyrophosphate strike on other “low EMF potential” substrates, such as stainless steel and titanium.
- On-going work will continue with ion exchange manufacturers to find the most effective resin options to remove weakly complexed copper from rinse water.
About the authors
Richard DePoto is Business Development Manager with Uyemura International Corporation Southington, CT. Richard is currently focusing on technical PM surface protection, hard silver as a replacement for gold, and processes for medical device and other applications requiring an ultra high purity surface. He holds a Bachelor of Science degree in Chemical Engineering from Stony Brook University in New York. Richard has been in involved with electroless and electroplating for the past 30 years and has held numerous positions in engineering, manufacturing and applied development, most notably AMP-AKZO Corporation
Douglas Duda is Laboratory Manager at Uyemura International in Southington, Connecticut.



































