Lithium-ion batteries have wide applications owing to their high energy density and relatively low weight.
With the rising popularity of electric vehicles and the need for renewable energy storage solutions, the demand for lithium-ion batteries is expected to increase. In this regard, it is necessary to switch away from rare and expensive metals currently used in the production of these batteries.
Sulfur has been identified as a potential alternative to nickel and cobalt, materials currently used in cathodes for lithium-ion batteries. It is the tenth most abundant element on earth, stores ten times as much capacity, and has an energy density that is 2–3 times higher than current lithium-ion battery cathodes. However, due to its insulating nature, it is often encapsulated in conductive polymer or carbon hosts, which lower the sulfur content and the charge-holding capacity of the battery.
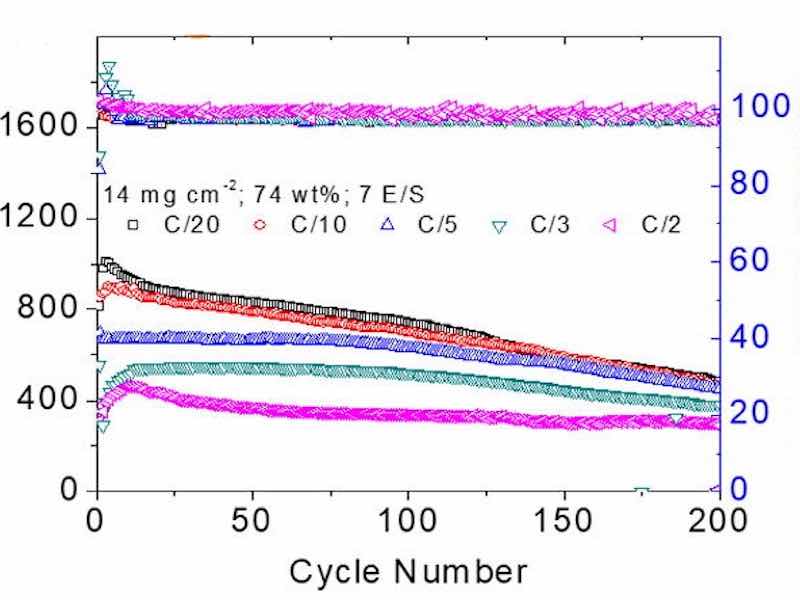
Now, in a study published in Chemical Engineering Journal on February 2022, researchers from National Cheng Kung University (NCKU), Taiwan, have developed a method to chemically coat sulfur particles with nickel, resulting in a nickel-plated sulfur nanocomposite for cathodes with a high sulfur content of about 74%.
"Besides its high electrocatalytic activity, nickel has a high conductivity, decreases cathode resistance when added to sulfur cathodes, increases polysulfide retention, and facilitates the redox reaction of the solid active materials. To adopt these material characteristics to improve lithium-sulfur electrochemistry, we applied the electroless-nickel-plated sulfur as the active material in an electrochemical sulfur cathode," says Dr. Sheng-Heng Chung, an assistant professor in the Department of Materials Science and Engineering, NCKU, and the study's lead author.
In this method, sulfur particles are initially prepared for nickel deposition through a sensitization and activation process, which involves adding sulfur particles to tin and palladium chloride-containing solutions. During sensitization, sulfur particles absorb tin (Sn2+) ions, which are then oxidized in the activation process, to form a (Pd0) activated layer on sulfur particle surfaces. Subsequently, nickel ions from a nickel salt are reduced on sulfur particle surface to form a nickel-plated sulfur nanocomposite with high sulfur content. The cathode was formed by dispersing the nanocomposite in a liquid electrolyte and drop-casting onto a current collector. The high conductivity of nickel and the favorable charge-holding capacities of sulfur resulted in a cathode with superior electrochemical characteristics.
"The lithium-sulfur cells with our nickel-plated sulfur energy-storage material attained a high specific areal capacity of 14–7 mA∙h cm-2 and high energy density of 28–13 mW∙h cm-2, values much higher than those required for powering electric vehicles and for commercial lithium-ion battery cathode," says Dr. Chung.
The nickel coating also strongly adsorbed the polysulfides formed during the intermediate discharge and charge stages of the battery. By preventing the active material from dissolving into the electrolyte, the addition of the coating improves the stability and cycling performance of lithium-ion batteries. This plating process can be extended to other metals to create cheap and high-capacity batteries with multiple applications.
"The nickel-plated sulfur energy-storage materials could be commercially available soon. In the future, the devices and battery power plant would have advanced lithium-sulfur cells with ten times higher capacity and 1/50 lower cost," says Dr. Chung.