When a plating shop lost its foreman, he apparently kept most of the important information on their plating solutions to himself.
 Frank AltmayerIn the meantime, they had a dickens of a time with their two bright nickel plating solutions. It did not help that one of these had essentially spilled onto the floor recently and had been recovered from their containment system.
Frank AltmayerIn the meantime, they had a dickens of a time with their two bright nickel plating solutions. It did not help that one of these had essentially spilled onto the floor recently and had been recovered from their containment system.
The shop asked me to get these solutions back to good working order, and the first thing we did was analyze samples from both nickel-plating solutions. We found that the basic chemistry was in order:
The two nickel plating solutions yielded the following analytical results (oz/gal or as indicated):
| Tank 38 | Tank 39 | |
| Metallic Nickel | 11.18 | 11.02 |
| Nickel Sulfate | 43 | 39.82 |
| Nickel Chloride | 6.34 | 8.58 |
| Boric Acid | 6.85 | 7.75 |
| pH | 4.7 | 4.2 |
| Copper (ppm) | 2.6 | 9 |
| Zinc (ppm) | 54.5 | 67.5 |
Each solution was heavily contaminated with nickel and some copper. The Hull Cell panels plated from these solutions had the following appearance(s):
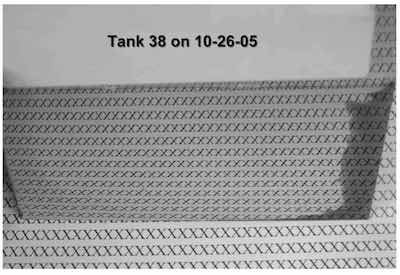
- Tank 38: The Hull Cell indicated a minor level of organic contamination that caused burning in the high current density area. The low current density was dark due to zinc contamination. The panel showed a broad, bright range. This solution requires low current density dummying to bring the zinc down below about 20 ppm.
- Tank 39: The deposit from this solution was very brittle and burned in the high and medium current density ranges, indicating a very high level of organic contamination. The low current density area was dark, confirming heavy metal (zinc, copper) contamination, which requires low current density dummying (1–5 A/ft2) to remove.
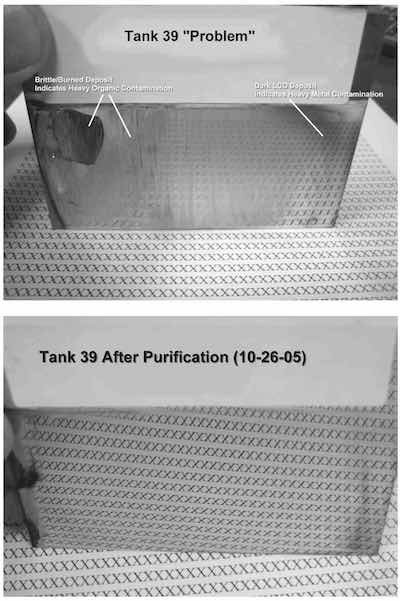 After an initial peroxide-carbon treatment, there was some improvement in the bright range, but the deposit was still brittle and burned in the high current density range. A second carbon treatment yielded further improvement. This second carbon treatment involved the use of potassium permanganate plus peroxide (diluted 1/100 before adding) prior to carbon treatment.
After an initial peroxide-carbon treatment, there was some improvement in the bright range, but the deposit was still brittle and burned in the high current density range. A second carbon treatment yielded further improvement. This second carbon treatment involved the use of potassium permanganate plus peroxide (diluted 1/100 before adding) prior to carbon treatment.
We recommend that this solution undergo a full purification treatment of peroxide and permanganate, plus high pH treatment.
The photos show Hull Cell panels plated in both tanks. Below is detailed information on purification procedures originally authored by Louis Geanelos.
Step-by-step Purification Procedures
Oxidation Treatments
The following treatments should be given only after bench plating tests have shown the need and determined the quantities to be used. All precautions should be observed carefully to ensure that oxidizing materials are not present in the treated plating solution.
Treatment with Hydrogen Peroxide and Carbon
- Pack filter with activated carbon using about 2.5 g/L (2 1b/100 gal) of plating solution when possible. Transfer the solution to the treatment tank by pumping through the packed filter. This is done to remove part of the anti-pitter.
- Allow solution to cool to 38–43°C (100–110°F) and adjust pH to 3.5.
- Add slowly, with agitation, the required amount of 100-volume hydrogen peroxide (30 percent). Depending on the severity of the treatment, this quantity will vary from 0.5–3 mL/L (0.5–3 gal/1000 gal) of plating solution.
- Agitate and maintain temp at 38–43°C (100–110°F) for at least two hr.
- Raise the temperature to 70°C (160°F) and maintain the temperature for at least two hours to remove excess peroxide. (Important!)
- Add 4.8–7.2 g/L (4 –6 lb/100 gal) activated carbon and agitate for at least two hours with a temp of 65–70°C (150–160°F).
- Allow the solution to settle thoroughly; meanwhile, clean the tank and anode bags. Replace worn-out bags and anodes.
- Filter the solution into a clean plating tank.
- Add additional agent(s) in proper quantities and resume plating operations after checking and adjusting pH and temp.
Combination Treatment-High pH, Peroxide, and Carbon
 Follow step-by-step procedure points 1 through 6 as outlined under “Treatment With Peroxide and Carbon,” then follow Steps 3 through 10 as directed under “High pH Treatment”.
Follow step-by-step procedure points 1 through 6 as outlined under “Treatment With Peroxide and Carbon,” then follow Steps 3 through 10 as directed under “High pH Treatment”.
Special Precautions for Peroxide and Carbon Purification of Nickel Solutions
The following points are discussed to enable the operator to perform a treatment with a greater degree of effectiveness and reduce the possibility of an unsatisfactory deposit due to improper purification of the solution.
- Step 1 is considered essential, and failure to perform this part of the treatment may lead to incomplete removal of impurities.
- The conditions specified under Step 2 are important and should be followed as closely as possible. The low temperature is required to prevent the decomposition of the hydrogen peroxide until it has served its oxidizing function. For the same reason, a pH of 3.5 is best. A low pH makes it difficult to remove the peroxide under Step 5, and a pH that is too high increases the decomposition rate of the peroxide.
- The complete removal of hydrogen peroxide under Steps 5 and 6 is of utmost importance. Failure to remove the peroxide completely will cause subsequent operating difficulties due to the action of any residual peroxide on the addition agents. To ensure complete removal, the temperature should be maintained at 65°C (160°F) until a test shows the absence of residual hydrogen peroxide.
Test for Presence of Peroxide in Nickel Plating Solutions
- Dissolve 5g of potassium iodide in 100 ml water. Add 5 g of soluble starch and heat until the starch is completely dissolved.
- Place one drop of plating solution on filter paper.
- Place two drops of the iodide-starch indicator over the solution spot on the filter paper.
- Observe color. If a blue color develops within 5 sec, peroxide is present.
Copper interferes at concentrations of 10 ppm; however, the reaction at these concentrations is slow, and no color will develop in 5 sec. Most nickel baths contain less copper than this amount. The starch-iodide reagent is not stable but will last about a week, depending on the conditions of storage. It is suggested that the reagent be prepared fresh as needed.
Oxidation with Permanganate
This treatment removes many organics that activated carbon alone or peroxide-carbon treatments do not. Generally, a solution of potassium permanganate is added, followed by carbon treatment and then high pH treatment. The amount of potassium permanganate used will vary with the degree of contamination and should be determined on the basis of laboratory testing. In this case, the plating solution is titrated with permanganate at Step 4 until the appearance of a permanent pink due to excess permanganate. A quantity less than this is then used.
The following treatment procedure should be used:
- Transfer the solution to a clean, suitably-lined storage tank.
- Adjust the pH of the solution to any point between 3.0 and 3.5.
- Adjust the temp of the solution between 65°C and 75°C (150°F and 170°F).
- Dissolve the required amount of potassium permanganate in an appropriate amount of warm water. About 500g/4 liters (1 lb/gal) of permanganate will suffice. Add slowly to the plating solution while stirring. Continue agitation for two hr.
- Continue as directed under “Combination Treatment With Carbon and Nickel Carbonate.” Due to the large volume of insoluble material that is filtered out, it is frequently easier to perform two filtrations, i.e., treatment with permanganate plus carbon and then with nickel carbonate.
Carbon Treatment
The following procedure should be used to eliminate contamination caused by oils, greases, buffing compounds, and other organic impurities:
- Transfer the solution to a clean and suitably-lined storage tank.
- Adjust the pH of the solution to 3.0–3.5.
- Adjust temp of solution to 65–75°C (150–170°F).
- Add 4.8 to 9.6 g/L (4–8 lb/100 gal) of activated carbon. 4.8–6.0 g/L of carbon is usually sufficient, but in cases of badly contaminated solution, 9.6 g/L or even more may be necessary. Two separate carbon treatments using 4.8 g/L (4 1b/100 gal) are better than one treatment using 9.6 g/L (8 lb/100 gal), but two filtrations are involved.
- While the is maintained, carbon should be stirred in carefully for at least 2 hr. Allow the solution to settle for an hour or more. Clean plating tank and anode bags; replace worn-out bags and anodes.
- The filter goes back into the cleaned plating tank using the pre-coated filter. Draw a solution from near the top to avoid picking up loose quantities of carbon sludge, which could restrict the flow or even stop up the filter.
- It is advisable to maintain an even rate of flow by adding about 1.2 g/L (1 lb/100 gal) of “filter aid.” This should be added slowly in the storage tank near the suction hose as the level of the solution is reduced.
- Adjust solution level and pH to optimum plating range. The pH can be raised by circulating the solution through a filter unit that has been packed with nickel carbonate and a “filter aid.” The pH can be lowered by adding diluted sulfuric acid.
- Adjust and add additional agent(s) as required.
- Resume plating operations.
High pH Treatment With Nickel Carbonate
If a high pH is required for the removal of metallic impurities, the following procedure should be followed:
- Transfer the solution to a clean and suitably-lined storage tank.
- Adjust temp to between 65-75°C (150-170°F).
- Prepare a slurry of nickel carbonate and water on the basis of 1 kg of dry nickel carbonate to one Liter of water with 7 ml of the antipitter used in the nickel bath (8 lbs of dry nickel carbonate to one gal of water with 1/4 oz anti-pitter). Stir this mixture, preferably with a mechanical stirrer, until completely dispersed. Do not add dry nickel carbonate directly to the solution in the storage tank. Always use a slurry.
- Slowly add the nickel carbonate slurry to the heated solution in the storage tank, stirring constantly. The amount of nickel carbonate added will vary with the original pH of the solution, but 6 g/L (5 lb/100 gal) of dry nickel carbonate to plating solution is usually sufficient.
- While the temperature is maintained, the solution should be stirred for about one hour or more. Then, check the pH of a filtered sample of solution and adjust it again with a nickel carbonate slurry until the pH is at least 5.2.
- Allow the solution to settle for at least eight hr, preferably overnight. Clean plating tank and anode bags; replace worn-out bags and anodes.
- The filter goes back into the cleaned plating tank using the pre-coated filter. Draw solution from near top to avoid flow restriction or filter stop-up.
- It is advisable to maintain an even rate of flow by adding about 1.2 g/L (1 lb/100 gal) of “filter aid” to the plating solution. This should be added slowly to the storage tank near the suction hose as the level of the solution is lowered.
- Reduce pH to the operating range with diluted sulfuric acid. 10. Adjust temp and addition agent(s). Resume plating.
Combination Treatment with Carbon and Nickel Carbonate
Treatments involving both activated carbon and nickel carbonate are ideally performed separately and in the order given. Lacking sufficient time to perform both treatments, a satisfactory compromise can be used when the solution is first treated with activated carbon followed by nickel carbonate. The following procedure should be used: Perform Steps 1 through 5 under “Carbon Treatment”. Instead of allowing the solution to settle under Step 5 above, perform Steps 3 through 10 as directed under “High pH Treatment.”
Frank Altmayer is a Master Surface Finisher and an AESF Fellow who is the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in metal finishing. He was the recipient of the AESF Past Presidents Award, NAMF Award of Special Recognition, AESF Leadership Award, AESF Fellowship Award, Chicago Branch AESF Geldzahler Service Award, and NASF Award of Special Recognition.



































