Electrodeposition of chromium (Cr) and nickel (Ni) and their alloys have been subjects of interest for a long time.
Owing to their special features such as high corrosion resistance, good wear resistance, lustrous nature etc., Cr and Ni electrodeposit continue to be the subject of investigation and further development, and improvements in their qualities and application remain one of the aims.
This paper reviews the advances made so far in Cr and Ni electrodeposited coatings, with special emphasis on showing the potential of the process for achieving high-quality coatings. Furthermore, this review focuses on the mechanisms involved in Cr and Ni electrodepositions, with the aim of understanding the basis and manipulating the processes to produce coatings with excellent features and high-end usage.
The issues encountered in the electrodeposition processes and products, as well as proffered solutions via research and development, were also addressed. Finally, long-term prospects and applications of these coatings were discussed to provide powerful and complementary toolkits for engineering applications while enabling future advances in critical aspects identified.
1. Introduction
Electrodeposition, also known as electroplating, is an uncomplicated and versatile method to produce coatings under ambient temperature and normal pressure, with benefits of low cost and a high deposition rate using the mechanism of hydrolysis. Consequently, this technique presents substantial advantages compared to other technologies for coating, such as vapor deposition, physical coating spraying, plasma nitriding, roll-to-roll coating, and others [1,2,3,4]. Apart from the benefits listed, electrodeposition technology has an intriguing ability to coat substrates of complicated geometrical forms and large surfaces with homogeneity of thicknesses ranging from nanometers to several tens of micrometers [2]. Based on these attributes, electrodeposition has gained significant relevance as an attractive surface-finishing technique.
Electrodeposition studies have had a long tradition; however, the earlier works on electroplating and the prevailing theoretical description of the phenomenon, hitherto treated in a rather piecemeal way, were initially expressed in an organized and extensive manner by Brenner in his two-volumes book Electrodeposition of alloys in 1963 [5,6]. Historically, the main thrust in the investigation into electrodeposition has been two-fold: Firstly improving corrosion resistance and, secondly, improving the wear resistance and mechanical properties of materials. In more recent times, these two demands continue to be of prominence; notwithstanding, a heightened research effort has gone into the study and development of processes for other areas of applications, such as magnetic properties [7,8,9,10,11,12,13,14,15,16,17,18,19] and catalysis, hydrogen permeability and separation [20,21,22,23,24,25,26,27,28,29], membrane technology, electronic devices, metallization of non- and semiconductors, and fuel cells [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Furthermore, electrodeposition is one of the most convenient methods to obtain metal/alloys of high melting points, such as chromium (Cr, 1907 °C), nickel (Ni, 1455 °C), and molybdenum (Mo, 2623 °C) [54,55], even at ambient temperatures, of which the first two are of interest in this review. Chromium and nickel deposits rank amongst the most widespread metal coatings for functional and decorative purposes, which have found applications in diverse industries owing to their outstanding properties, such as high corrosion resistance, wear resistance, and hardness.
In the case of chromium, as shown in Figure 1 (left), because of its lustrous, hard, excellent corrosion resistance and low friction, its electrodeposition is used in extending the service life of a number of durable goods, such as kitchen utensils (gas burners, washing machine and dryer dials, and heating grates); bathroom appliances (bathroom faucets and door handles); and automotive parts (wheel trim, bumpers, license plate frames, and headlights) [56]. Meanwhile, for nickel coating, its advantageous properties, such as impressive corrosion resistance, moderate hardness, wear resistance, good radiation resistance, and electrical conductivity, have made it suitable for applications in various sectors, as depicted in Figure 1 (right). For instance, the excellent electrical conductivity property of nickel makes it useful for battery and generator applications, while its hardness and durability make it ideal for equipment employed in harsh conditions, such as pump mixers, valves, shafts, and heat exchangers used in the oil and gas industry. Additionally, the automobile industry oftentimes uses nickel in the electroplating of gears, steering shafts, motor housing starters, fuel system components, and break caliper pins [57].
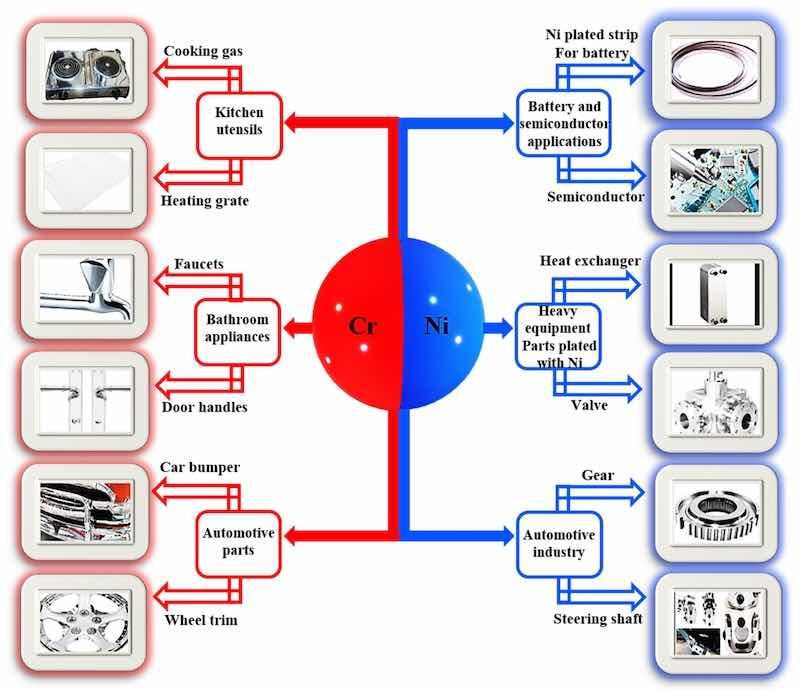 Figure 1. A schematic of the diverse applications of Cr and Ni electrodeposited coatings.
Figure 1. A schematic of the diverse applications of Cr and Ni electrodeposited coatings.
Over the years, several investigations have been carried out in developing electroplated Cr and Ni coatings for numerous industrial applications [58,59]. In regard to chromium, the hexavalent Cr process has been the main development technology for Cr electrodeposition [60,61,62,63,64]. Notwithstanding, substituting the hexavalent Cr process with the trivalent Cr process became an imperative and urgent issue in the early 2000s because of the carcinogenicity and toxicity of hexavalent Cr [58,65]. This replacement scheme and constant modification of the trivalent Cr bath are among the recent advances in chromium electrodeposition. However, it is important to note that the trivalent Cr process is more difficult to develop when compared to the hexavalent Cr process, and many parts of detailed information are still patented. From the literature gathered, it is conventionally impossible to reduce the trivalent Cr ion directly on the cathode material without complexants [58,59,66,67,68,69,70,71]. In other words, the presence of complexants, usually an organic liquid, instigates the complexation of trivalent Cr and subsequent reduction to metallic Cr [66,67,68,69]. The reason for this is because the substitution of hydrated water molecules by the organic ligands destabilizes the stable (Cr(H2O)6)3+ complex to an electroactive ligand-containing Cr (III) complex, which, in turn, makes the reduction of Cr (III) species feasible. Furthermore, in the presence of organic complexants such as glycine, carbamide, urea, formate, and oxalate, carbon (C) is co-deposited with chromium [65,66,67,68,69,70], and the resulting Cr-C deposits are amorphous or nanocrystalline [72,73]. Based on previous studies, it has been pointed out that the microstructure and mechanical properties of chromium coatings obtained by hexavalent and trivalent procedures are not identical but quite comparable [60,65].
With respect to Ni electrodeposition, Ni coatings have gained interest over the years as well, and in more recent times, the focus has been on improving the quality of Ni coatings with the incorporation of other element(s) to account for the limitations of nickel, such as low corrosion resistance in an aggressive chlorine environment, insufficient mechanical strength, and wear resistance in applications where such properties are in high demand [74]. For example, Jinlong L. et al. [75] studied the effect of a tungsten (W) addition to Ni electrodeposition on the mechanical properties and corrosion resistance of the coating. Their results revealed that the Ni-W coating showed a higher corrosion resistance than the pure Ni electrodeposited coatings in 3.5% NaCl solution and borate buffer solution. They ascribed this phenomenon to the less cation vacancy in the passive film formed on the Ni-W electrodeposited coatings. Additionally, the Ni-W electrodeposited coating exhibited excellent and better tensile strength, yield strength, and considerable elongation than the pure Ni coating. Moreover, in search of a new material for coin blanks, Schweckandt D. et al. [76] synthesized Ni-Co electrodeposited coatings on brass. The findings of their study showed that incorporating Co into Ni in a ratio of Ni42Co58 produced an alloy with a suitable hardness, roughness, and conductivity very close to that of the coins in circulation. To date, research is still being undertaken on tailored Ni-X (X = Co, W, Cu, Mo, and P) bimetallic/multilayered coatings to suit different industrial purposes. For instance, the magnetic properties of nickel have been successfully improved by the addition of Co and Cu to suit applications such as biosensors, magnetic field sensors, and miniature devices [56,77,78]. In particular, Ni-Co electrodeposited coatings have been found to be better for applications such as electronics, computers, energy (lithium batteries), biotechnological applications, storage devices, etc. because of their satisfactory magnetic, chemical, mechanical, electrocatalytic, and physical properties [79,80,81,82].
This article critically reviews the mechanisms involved in Cr and Ni electrodepositions, the influence of the process parameters, and their modifications in relation to recent trends on the fabrication, microstructure, and engineering properties of Cr and Ni electrodeposited coatings. This review also focuses on the challenges encountered in the processing and production of electroplated Cr and Ni coatings, as well as the recently proposed solutions via research and development. Finally, long-term prospects and applications of these coatings are also considered with a view to providing powerful and complimentary toolkits for engineering applications while enabling future advances.
2. Mechanism of Cr and Ni Electrodepositions
The literature evidence [63,83,84,85,86] has proposed some conventional and detailed routes for Cr and Ni electrodeposition mechanisms, which will be dealt with in perspective in this section.
2.1. Cr Electrodeposition Mechanism
Generally, Cr electrodeposition in a trivalent Cr bath occurs in two basic consecutive steps, which are electrochemical reduction of the Cr3+ species to Cr2+ and, then, from the Cr2+ species to Cr (s), as shown in Equations (1) and (2), respectively.
Cr3+ + e− → Cr2+ (1)
Cr2+ + 2e− → Cr (s) (2)
Corresponding to the two-step Cr ion reduction in the electrodeposition process of Cr is the hydrogen gas evolution reaction also occurring at the cathode, as presented in Equation (3) [86].
2H2O + 2e− → H2(g) + 2OH− (3)
In specific, Cr ions in aqueous trivalent bath exist in diverse complex forms, such as [Cr(H2O)6]3+, [(H2O)4Cr(OH)(OH)Cr(H2O)]4+, [Cr(H2O)5Cl]2+, [(H2O)5Cr(OH)Cr(H2O)5]5+, [Cr(H2O)5 (OH)]2+, [Cr (H2O)4Cl2]3+, etc. [63,86]. Any of these complex forms undergo reduction to form an intermediate complex that is adsorbed. For example, Khani H. and Brennecke J. [86] reported that trivalent chromium chloride-hydroxide is reduced to bivalent chromium chloride-hydroxide, [Cr (H2O)4Cl2]2+, during the first step reduction in Cr electrodeposition, as represented in Equations (4) and (5), respectively.
CrIII(H2O)4Cl2 + e− → CrII (H2O)4Cl2 (4)
CrII(H2O)4Cl2 + OH− → CrII(H2O)4(OH)Cl + Cl− (5)
The first step reduction reaction is subsequently followed by the reduction of the adsorbed intermediate complex to metallic chromium or Cr(OH2), as given in Equations (6) and (7), respectively. The last-mentioned will afterward be reduced to metallic chromium according to Equation (8) [87,88,89].
CrII (H2O)4(OH)Cl + 2e− → Cr0 + 4H2O + Cl− + OH− (6)
CrII (H2O)4(OH)Cl + OH− → Cr(OH)2 + 4H2O + Cl− (7)
Cr(OH)2 + 2e− → Cr0 + 2OH− (8)
Meanwhile, in the scenario where organic ligands are present, the H2O, OH, or Cl− species of the inorganic Cr3+ complexes may be substituted by the organic ligands to produce organo-chromium complexes such as [Cr(H2O)5(HCOO)]2+, [Cr(H2O)5(CH3COO)]2+, and [Cr(EDTA)]−. Based on this principle, Song Y. and Chin D. [63] proposed the mechanism of Cr electrodeposition from a trivalent Cr bath containing formate and acetate in their study. According to their findings, CrII(H2O)5Cl ions are converted to CrII(H2O)5COOH and CrII(H2O)5OOCCH3 by the following equations represented in Equations (9) and (10), respectively.
CrII (H2O)5Cl + HCOO− → CrII(H2O)5 HCOO + Cl− (9)
CrII (H2O)5Cl + CH3COO− → CrII(H2O)5 CH3COO + Cl− (10)
Subsequently, the reduction of the organo-chromium complexes to metallic chromium occurred following the reaction steps presented in Equations (11)–(14).
CrII(H2O)5 HCOO + e− → CrI(H2O)5 HCOO (11)
CrII(H2O)5 CH3COO + e− → CrI(H2O)5CH3COO (12)
CrI(H2O)5 HCOO + 2e− → Cr (s) + 5H2O + HCOO− (13)
CrI(H2O)5CH3COO + 2e− → Cr (s) + 5H2O+ CH3COO− (14)
Thus, it was observed that the reduction reaction of a complex Cr3+ to a complex Cr2+ ion is the predominant step in the trivalent Cr deposition process. However, only a small fraction of [Cr(H2O)5(HCOO)]2+ and [Cr(H2O)5(CH3COO)]2+ formed at the cathode is further reduced through Equations (11)–(14). Some of them are conveyed by electrolyte convention to the bulk solution. This fact agrees with the experimental results of the rotating disk electrode in their study, which showed that only 4% of the trivalent chromium ions in the electrolytic bath participated in the overall electrodeposition reactions of Equations (11)–(14) [63].
In summary, the sequence of electrodeposition of Cr involves two basic reduction steps, and the rate of deposition is controlled by the diffusion of the complex Cr3+ ions to the cathode surface. Figure 2 shows the schematic explanation of the mechanism of Cr electrodeposition.
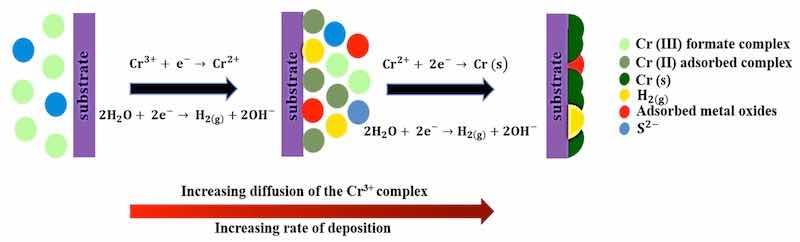 Figure 2. A schematic explanation of the mechanism of Cr electrodeposition.
Figure 2. A schematic explanation of the mechanism of Cr electrodeposition.
2.2. Ni Electrodeposition Mechanism
The mechanism of Ni electrodeposition generally involves two consecutive one-electron charge transfers and the participation of an anion in the formation of an adsorbed complex. Equations (15)–(17) give the representative reactions involved in the Ni deposition mechanism.
Ni2+ + X− →NiX+ (15)
NiX+ + e− → NiX(ads) (16)
NiX(ads) + e− →Ni + X− (17)
In these reactions X− denotes any of the OH−, SO42−, or Cl− anions or an organic ligand [90,91,92]. However, previous studies have reported that if three anions (OH−, SO42−, or Cl−) are present in an electrolytic bath (for example, a Watts bath containing NiSO4, NaCl, and H3BO3), the anion X− is usually Cl−, and the rate-determining step is the reaction in Equation (16), i.e., the first electron transfer step [90,92].
Simultaneously, the discharge of hydrogen occurs in several steps, of which two alternative mechanisms are possible [90]. Firstly, the hydronium cation (H3O+) is desolvated and partially discharged at the electrode surface, as in Equation (18). Alternatively, the hydrogen atoms combine in the adsorbed state to form adsorbed molecules, which is depicted in Equation (19).
H3O+ + e− → H(ads) + H2O (18)
2H(ads) → H2(ads) (19)
Any of these first steps is subsequently followed by the final step in the hydrogen discharge mechanism, which is the desorption of adsorbed hydrogen molecules as bubbles given in Equation (20).
NH2 → (H2)n (20)
As pointed out earlier, since the nickel deposition process and the hydrogen evolution process occur simultaneously, there are possibilities of these mechanisms interacting with each other during the deposition process. The effect of such an interaction will be elucidated in the next section.
Some authors have proposed other mechanisms of Ni deposition that are slightly different from the conventional mechanism. For instance, Supicova M. et al. [93] gave a deeper insight into the mechanism of electrode reaction during Ni deposition when they studied the electrolytic deposition of Ni onto a paraffin-impregnated graphite electrode (PIGE) at pH 2 and 4 from a chloride electrolyte. Their findings stipulated three steps in the deposition mechanism of Ni from the chloride electrolyte, which are: (i) a chemical reaction preceding an electrochemical reaction, (ii) the occurrence of surface reactions with the adsorption of intermediates onto the PIGE, and (iii) a reaction of the electroactive substance transported to the electrode by diffusion. Although they pointed out from elimination voltammetry the importance of a kinetically controlled adsorption/desorption process in the nickel deposition mechanism, which is indicated in Equations (15)–(17) (the conventional mechanism). In the mechanism proposed by Gomez E. et al. [94], the reduction of Ni(II) (as shown in Equation (21)) was the first step involved in the deposition of Ni, which is followed by one or more steps that lead to the final deposition.
Ni(II) + e− → Ni(I) (21)
This simple scheme may apply at potentials close to those after the start of the deposition until the maximum potential of the reduction peak is reached. At more negative potential, the first step was maintained but was followed by a possible disproportion reaction of Ni(I), as presented in Equation (22).
Ni(I) + Ni(I) →Ni(0) +Ni(II) (22)
Furthermore, under these conditions, a simultaneous reaction may occur between Ni(I) and water, which is represented in Equation (23).
Ni(I) + H2O → NiOH(I) + H (23)
This reaction explains the production of hydrogen during the Ni deposition.
3. Influence of Electroplating Parameters on the Cr and Ni Coatings
3.1. Current Density
One of the key parameters in electroplating is the current density. Generally, it has been known that the electroplating process relates to nucleation and growth. At the high current density, the nucleation rate is greater compared to the growth [95]. Therefore, the grain size decreases with the increasing current density. This phenomenon may not be totally true in all cases, as Ebrahimi et al. reported that a high current density resulted in an increased grain size of Ni deposits owing to hydrogen evolution in their study [96]. Additionally, it was reported that the current density results in a different effect on the grain size, depending on the types of baths used [97,98]. According to Faraday’s law, the changes in the current density lead to an increase in coating thickness theoretically. However, Bayramoglu et al. [99] reported that the Cr coating thickness decreases beyond the optimal current density due to the hydrogen absorbed by the cathode, leading to less deposition of Cr on the substrate. Therefore, the current density singly or intertwined with other factors can have diverse effects on coatings, depending on the conditions involved. By this, it is pertinent to suggest that investigators should not just assume the effect of current density based on the previous literature, but make painstaking and informed decisions based on thorough investigations in relation to their condition of electrodeposition.
3.2. Temperature
![Figure 3. Effects of temperature and current density on the appearance of chromium electrodeposits. Reprinted with permission from [69].](/images/images/whitepapers/advancescrniplating/3.jpg) Figure 3. Effects of temperature and current density on the appearance of chromium electrodeposits. Reprinted with permission from [69].Another important parameter is the temperature. The temperature influences the grain size, crystallographic orientation, and composition in the deposit. Gómez et al. [100] reported that the grain size increases with the increase of the temperature in electrodeposition solution. Jinlong et al. [101] revealed that the X-ray diffraction results for electroplated Ni are different, depending on the bath temperature. Especially, the temperature affects the texture and the defect density, leading to changes in the hardness of the Ni layer [102]. According to previous studies for Ni layers on the Cu alloy substrate at 20°C –50°C, the temperature affects the crystallographic orientation and the surface morphology, resulting in different corrosion resistance [103]. Additionally, the Cr coating thickness increases with increasing the electrolyte temperature, because the higher temperature results in an increase in conductivity and the diffusion rate of ions, thereby providing an increased rate of metal dissolution at the anode and deposition at the cathode [99]. However, this may change as the temperature increases above certain temperature ranges [69]. According to previous studies for Cr coatings, when the electrolyte temperature is above 70°C, a crack-free Cr layer is deposited on the substrate [104,105]. Sohi et al. [69] showed in their study using the Hull cell test the relationship between the effects of the current density and temperature in the Cr coatings. As represented in Figure 3, Cr electrodeposits with matte and milky appearances were crack-free, while others, especially the bright deposits, had a considerable number of cracks. Their findings also proposed that the optimum current density and temperature for obtaining a crack-free coating were 85 A/dm2 and 85°C, respectively. On the other hand, the optimum conditions for obtaining a hard chromium deposit were 45 A/dm2 and 55°C. Therefore, the purpose of the coating determines the conditions to apply during the electroplating process.
Figure 3. Effects of temperature and current density on the appearance of chromium electrodeposits. Reprinted with permission from [69].Another important parameter is the temperature. The temperature influences the grain size, crystallographic orientation, and composition in the deposit. Gómez et al. [100] reported that the grain size increases with the increase of the temperature in electrodeposition solution. Jinlong et al. [101] revealed that the X-ray diffraction results for electroplated Ni are different, depending on the bath temperature. Especially, the temperature affects the texture and the defect density, leading to changes in the hardness of the Ni layer [102]. According to previous studies for Ni layers on the Cu alloy substrate at 20°C –50°C, the temperature affects the crystallographic orientation and the surface morphology, resulting in different corrosion resistance [103]. Additionally, the Cr coating thickness increases with increasing the electrolyte temperature, because the higher temperature results in an increase in conductivity and the diffusion rate of ions, thereby providing an increased rate of metal dissolution at the anode and deposition at the cathode [99]. However, this may change as the temperature increases above certain temperature ranges [69]. According to previous studies for Cr coatings, when the electrolyte temperature is above 70°C, a crack-free Cr layer is deposited on the substrate [104,105]. Sohi et al. [69] showed in their study using the Hull cell test the relationship between the effects of the current density and temperature in the Cr coatings. As represented in Figure 3, Cr electrodeposits with matte and milky appearances were crack-free, while others, especially the bright deposits, had a considerable number of cracks. Their findings also proposed that the optimum current density and temperature for obtaining a crack-free coating were 85 A/dm2 and 85°C, respectively. On the other hand, the optimum conditions for obtaining a hard chromium deposit were 45 A/dm2 and 55°C. Therefore, the purpose of the coating determines the conditions to apply during the electroplating process.
3.3. pH
pH is another key factor in the electroplating process. pH affects the current efficiency and residual stress of coatings. At a high pH, not only the current efficiency but also the impurities and residual stress also increase. At a low pH, the current efficiency decreases, while the residual stress increases [106]. Thus, researchers studied to find the optimum pH value. Mandich et al. [107] reported that a pH of 4.2–4.5 is an optimized condition in the Watts nickel bath, considering the current efficiency and brightness. Additionally, Haque et al. [108] reported that the optimum pH value in a trivalent chromium bath was 3 for a high current efficiency. Protsenko et al. [109,110] reported that the optimum pH value was 1.5 in a sulfate trivalent chromium bath, because the crack was formed along the edge above pH 1.7.
3.4. Type of Current
In the process of electroplating, various types of currents are used, such as direct current (DC), pulsed current (PC), and pulse reverse current (PRC). The DC method most often has disadvantages in film defects, such as porosity, poor adhesion, and slow deposition [111]. In order to overcome these disadvantages, PC and PRC techniques have been developed [112,113]. In the PC, the cathodic current changes periodically between positive and zero values, while the cathodic current is altered between positive and negative values in the PRC [111]. According to previous studies, electrodeposition by the PC method leads to finer grain sizes and improved substrate adhesion [114]. Additionally, Nasirpouri et al. [111] reported that Ni film electrodeposited from a Watts bath using PC and PRC techniques showed high cathodic efficiency because of the small amount of hydrogen evolution compared to that using DC. Chung et al. [115] reported that the stiffness of the Ni layer electrodeposited by the PC method is improved compared to that by the DC method, because Ni diffuses into the surface defects during the off time. Ni electroplated by the PC and PRC techniques revealed a greater uniformity of electrolytic deposits and smaller grain sizes [116]. In addition, Ni electrodeposited by PC in the Watt bath containing saccharin indicated improved corrosion resistance and hardness compared to that by DC [117]. According to the studies for Cr deposits, the crack-free hard chromium coatings can be deposited by PC techniques, which showed enhanced corrosion resistance [118,119,120]. Additionally, the PC technique is widely used in the Cr deposit, because the Cr deposit by PC has an advantage, such as high throwing power and low residual stress [121].
3.5. Additives
Various additives are used to improve the coating properties, such as surface roughness, corrosion resistance, wear resistance, and current. According to previous studies, the organic additive could improve the structure and induce the smooth surface of deposits [117]. In addition, the grain size of Ni electrodeposits is affected by adding saccharin [122]. Likewise, Meng et al. [123] reported that pyramidal islands appeared on the Ni coatings obtained from baths with different concentrations of phytic acid, with the finest island appearing when the phytic acid concentration was 0.2 g/L, as shown in Figure 4. This phenomenal change induces the formation of twin structures due to the lower stacking fault energy, which affects the corrosion resistance of the Ni coatings.
![Figure 4. SEM observations of the surface micro-morphologies of Ni coatings obtained from a bath with 0 g/L (a), 0.1 g/L (b), 0.2 g/L (c), and 0.3 g/L (d) phytic acid. Reprinted with permission from [123].](/images/images/whitepapers/advancescrniplating/4.jpg) Figure 4. SEM observations of the surface micro-morphologies of Ni coatings obtained from a bath with 0 g/L (a), 0.1 g/L (b), 0.2 g/L (c), and 0.3 g/L (d) phytic acid. Reprinted with permission from [123].
Figure 4. SEM observations of the surface micro-morphologies of Ni coatings obtained from a bath with 0 g/L (a), 0.1 g/L (b), 0.2 g/L (c), and 0.3 g/L (d) phytic acid. Reprinted with permission from [123].
Furthermore, the addition of rare earth compounds into the Ni electrolyte bath enhances the corrosion and wear resistance of the coating [124,125,126]. Especially, López et al. [127] reported that the addition of samarium affects the corrosion resistance, which is caused by the microstructure and formation of a passive film. According to the studies for Cr electroplating, the additives such as methyl-containing amines and polymers in the Cr electrolytic bath affect the current efficiency [128,129]. Additionally, Protsenko et al. [130] reported that the addition of sodium fluoride to the sulphate trivalent Cr bath improves the current efficiency, because fluoride ions accelerate the electrochemical process. Mehdipour et al. [131] reported that the corrosion behavior could be improved by adding the glycine into the trivalent Cr bath because of the low crack density. Additionally, the addition of polyethylene glycol to the trivalent Cr bath led to the improved corrosion resistance of Cr coatings [132].
4. Issues of Ni and Cr Electroplating
4.1. Electroplating Process
Retrospectively, several challenges have been identified in the electroplating processes involved in Cr and Ni electrodepositions. Consequently, research has been tailored towards addressing these challenges, some of which will be discussed in the next subsections.
4.1.1. Chromium Plating Process Issues
![Figure 5. pH effect of a trivalent Cr bath on the carbon content in electrodeposited Cr coating. Reprinted with permission from [83].](/images/images/whitepapers/advancescrniplating/5.jpg) Figure 5. pH effect of a trivalent Cr bath on the carbon content in electrodeposited Cr coating. Reprinted with permission from [83].The most important challenge that has affected the Cr electroplating over the years is the health implication of the conventional chromium hexavalent bath. Due to this health implication, the United State and European Union have banned the use of hexavalent Cr-bath since the year 2000 [133,134,135]. In order to resolve this issue, researchers proposed that the technologically feasible approach is by substituting the hexavalent Cr bath with a trivalent Cr bath, which is far less toxic [135,136]. The trivalent Cr bath may have been a successful replacement for the hexavalent Cr bath in regard to health implications; however, the electroplating process is not without its challenges, which, in the past and currently, are being investigated to resolve and improve. Some associated issues with trivalent Cr-bath are: (1) low current efficiency in comparison to the hexavalent Cr bath. According to Srimathi N. and Mayanna S. [137], the reduction of water into hydrogen with potential close to that of the reduction of Cr (III) to Cr (II) and to Cr (0) runs with very low overpotential on transition metal deposits, which leads to free hydrogen and hydroxide anions. This reaction of the reduction of water decreases the cathodic current efficiency, thereby making the precipitation of Cr (III) species somewhat difficult. (2) Difficulty in decomplexing the Cr (H2O)63+ ions owing to their classification as a strong Lewis acid that undergoes a rapid olation reaction between unprotonated forms in an aqueous medium, thereby annihilating Cr deposition [138]. Recent trends in Cr electroplating research have suggested some possible solutions to this associated phenomenal trait of the trivalent Cr bath. One of which is the investigation of chromium complexation with several ligands that are better complexing agents than water and effortlessly released from Cr (III) during the deposition process [58,59,138,139]. For instance, Philippe L. et al. [138] showed that it is possible to electrodeposit the Cr-Ni-Fe alloy with a thickness of approximately 23 µm and stressless deposit on a stainless steel and copper substrate with the trivalent Cr bath containing either dimethylformamide (DMF) or glycine complexant. Additionally, from their study, it was observed that glycine as a chromium complexant gave a higher reproducible deposit with a stable composition, surface state, and satisfactory mechanical properties than DMF. They ascribed the advantage of the glycine to the presence of a delocalized nitrogen lone pair.
Figure 5. pH effect of a trivalent Cr bath on the carbon content in electrodeposited Cr coating. Reprinted with permission from [83].The most important challenge that has affected the Cr electroplating over the years is the health implication of the conventional chromium hexavalent bath. Due to this health implication, the United State and European Union have banned the use of hexavalent Cr-bath since the year 2000 [133,134,135]. In order to resolve this issue, researchers proposed that the technologically feasible approach is by substituting the hexavalent Cr bath with a trivalent Cr bath, which is far less toxic [135,136]. The trivalent Cr bath may have been a successful replacement for the hexavalent Cr bath in regard to health implications; however, the electroplating process is not without its challenges, which, in the past and currently, are being investigated to resolve and improve. Some associated issues with trivalent Cr-bath are: (1) low current efficiency in comparison to the hexavalent Cr bath. According to Srimathi N. and Mayanna S. [137], the reduction of water into hydrogen with potential close to that of the reduction of Cr (III) to Cr (II) and to Cr (0) runs with very low overpotential on transition metal deposits, which leads to free hydrogen and hydroxide anions. This reaction of the reduction of water decreases the cathodic current efficiency, thereby making the precipitation of Cr (III) species somewhat difficult. (2) Difficulty in decomplexing the Cr (H2O)63+ ions owing to their classification as a strong Lewis acid that undergoes a rapid olation reaction between unprotonated forms in an aqueous medium, thereby annihilating Cr deposition [138]. Recent trends in Cr electroplating research have suggested some possible solutions to this associated phenomenal trait of the trivalent Cr bath. One of which is the investigation of chromium complexation with several ligands that are better complexing agents than water and effortlessly released from Cr (III) during the deposition process [58,59,138,139]. For instance, Philippe L. et al. [138] showed that it is possible to electrodeposit the Cr-Ni-Fe alloy with a thickness of approximately 23 µm and stressless deposit on a stainless steel and copper substrate with the trivalent Cr bath containing either dimethylformamide (DMF) or glycine complexant. Additionally, from their study, it was observed that glycine as a chromium complexant gave a higher reproducible deposit with a stable composition, surface state, and satisfactory mechanical properties than DMF. They ascribed the advantage of the glycine to the presence of a delocalized nitrogen lone pair.
Another proffered solution is the modification of the trivalent Cr bath. Barns S. et al. [140] observed that, by adding a saccharine additive, a sulfur-containing group in the trivalent Cr bath, the deposition rate, and current efficiency of Cr increased slightly. Based on their findings, the adsorption of SH-containing molecules decreased the polarizability of the cathode and therefore stimulated the electrochemical discharge reaction by preventing proton or hydrogen adsorption. In continuation of the modification of the trivalent Cr bath, Ghaziof et al. [83] reported that decreasing the pH of the trivalent Cr bath increases the carbon content during the deposition of Cr-C, as depicted in Figure 5, and a formate-urea containing the trivalent Cr bath used by Surviliene and colleagues [141] showed a positive increase in the current efficiency, while Chen W. et al. [65] noticed that the presence of thiosalicylic acid in the trivalent Cr bath evidently reduced the co-deposition of carbon (C), which, in turn, enhanced the crystallinity of the Cr-C-S deposit, as represented by the XRD result in Figure 6, which shows a decrease/disappearance of the broad hump peak at approximately 43° with an increase in the thiosalicylic concentration.
![Figure 6. XRD patterns of the deposits from the trivalent Cr bath (a) without thiosalicylic acid and containing (b) 0.00083, (c) 0.0025, (d) 0.005, and (e) 0.0083 mthiosalicylic acid. Reprinted with permission from [65].](/images/images/whitepapers/advancescrniplating/6.jpg) Figure 6. XRD patterns of the deposits from the trivalent Cr bath (a) without thiosalicylic acid and containing (b) 0.00083, (c) 0.0025, (d) 0.005, and (e) 0.0083 mthiosalicylic acid. Reprinted with permission from [65].Another challenging experience encountered in the Cr electroplating process is the presence of high stress at the interface of the Cr coating and substrate. Usually, this phenomenon occurs greatly during direct current electroplating. The high stress generated via direct current electroplating invariably leads to cracks in the chromium coatings [86].
Figure 6. XRD patterns of the deposits from the trivalent Cr bath (a) without thiosalicylic acid and containing (b) 0.00083, (c) 0.0025, (d) 0.005, and (e) 0.0083 mthiosalicylic acid. Reprinted with permission from [65].Another challenging experience encountered in the Cr electroplating process is the presence of high stress at the interface of the Cr coating and substrate. Usually, this phenomenon occurs greatly during direct current electroplating. The high stress generated via direct current electroplating invariably leads to cracks in the chromium coatings [86].
In resolving this challenge, the pulse current and pulse reversal electroplating methods which involve the rapid alternating of the electrical current between two different values, were proposed after a series of researchers found out that they could help to improve the quality of the electroplated film and release the internal stress built up during the fast deposition [142,143,144,145]. Leisner P. et al. [144] and others [146,147] found out that a combination of the short duty cycle and high frequency in pulse current electroplating mode could decrease the surface crack of Cr coating dramatically. However, their findings pointed out that, in order to maintain the constant effective current or potential, a high-performance power supply is required to provide a high current/potential and swift switch. According to Yeo S. et al. [120] and Landolt D. et al. [142], the pulse plating technique affects the microstructure and mechanical properties of deposits because of differences in mass transport, nucleation rates, and recrystallization behavior during the anodic and interruption times. Additionally, Leisner P. et al. [144] showed that crack-free Cr coating can be obtained by the pulse reversal plating method, of which they further proposed that the reoxidation of hydrogen from the surface during the anodic period is vital for producing a crack-free deposit. In particular, they pointed out that a specific anodic charge depending on the charge of the previous cathodic pulse is needed to obtain a crack-free coating and that the residual stress will be less at a more frequent current reversal.
4.1.2. Nickel Plating Process Issues
Conventionally, the electroplating process of nickel involves the chemical reduction of nickel ions to metallic nickel, which results from the presence of a reducing agent in the solution, as pointed out in Section 2. This process is usually associated with hydrogen evolution during the plating process and the formation of porous surfaces, which are challenging in terms of corrosion resistance and the deposition rate [148]. Cziraki A. et al. [149] and Ebrahimi F. et al. [96,150] reported that the increase in hydrogen evolution led to a localized increase in pH, thereby causing the formation of metal hydroxide near the cathode surface. As a consequence, the depletion of the metal ions near a cathode-electrolyte interface limits the current efficiency employed in direct current deposition. Additionally, they noticed that the adsorption of the hydrogen bubbles formed on the nickel deposit led to an increase in the roughness of the final deposits. In tackling these issues, Riedel R. et al. [151] and Hagiwara Y. et al. [152] proposed the use of surfactants as a wetting agent in Ni-plating baths. From their works, the presence of anionic and cationic surfactants proved to be effective in eliminating hydrogen from the surface of the substrate and in producing a pit-free nickel deposit. Tasuomi M. et al. [153] deposited a pit-free nickel coating by adding 150 ppm sodium dodecyl sulphate in the plating bath, while Yu Y. et al. [154] revealed that the addition of additives significantly influenced the anodic oxidation of the reducer, to which they ascribed this phenomenon as the main controlling step of the plating process. Their findings also showed that the higher the anodic oxidation current, the higher the oxidation speed, which has a direct relationship with the deposition rate. Chen et al. [155] achieved a deposition rate increase of about 25% when 5 ppm of Tween 20 was added in the electrolytic bath and a smoother morphology by using a surfactant in the acidic hypophosphite bath.
In addition, pulse and pulse reverse current electrodepositions have been proposed over the last two decades as simple and economical methods of depositing porous-free, bulk, and nanocrystalline Ni coatings [156,157,158]. The advantage of pulse and pulse reverse plating methods over the direct current plating method is that, while a high current density can momentarily polarize the cathode for a given duration during the pause time, the concentration polarization reduces, and this results in a smooth and fine grain deposit [156].
4.2. Issues of Formed Cr and Ni Electroplated Coatings
4.2.1. Issues of Electroplated Cr Coating
Generally, the major challenge of electroplated Cr coating is the presence of cracks in the deposit owing to internal stress, as pointed out earlier. In this subsection, the cause and the formation mechanism of the crack in Cr coating is elucidated, as well as proposed solutions to producing crack-free Cr coatings.
Conventionally, the crack formation in Cr coating can be explained by the crystallization mechanism of the electrodeposited Cr. As identified previously, electroplating of Cr is usually associated with a low current efficiency, which is accompanied by a considerably high level of hydrogen evolution. Subsequently, the reduced hydrogen is incorporated into the coatings and, as a result, leads to the formation of metastable hexagonal chromium hydride (β-Cr). Thereafter, the β-Cr decomposes into hydrogen gas and stable body-centered cubic (BCC) chromium (α-Cr) containing a residue of dissolved hydrogen. This decomposition occurs during the plating process and results in tensile stress, which, in turn, leads to the formation of cracks in the coating.
According to the literature [65], hydrogen incorporation plays an important role in the crack formation of Cr coatings in two ways, one of which is the inducement of compressive stress owing to the retention of the incorporated hydrogen in the deposit, and secondly, the inducement of tensile stress resulting from the release of the incorporated hydrogen from the deposit. However, apart from hydrogen incorporation, other theories have been proposed to be the cause of internal stress in Cr electrodeposited coatings, which are, crystallite joining, changes in foreign substances, excess energy, and lattice effects [65,159]. In regard to foreign substance effects, Chien W. et al. [65] proved that the incorporation of foreign substances such as carbon and sulfur originating from the complexants and thiosalicylic acid apparently influenced the internal stress of the Cr deposit formed in different ways. While the incorporation of sulfur reduced the internal stress, a vice versa phenomenon was observed for carbon incorporation. The drawback of the conventional mechanism of Cr crystallization is that it fails to explain the presence of a very strong texture in Cr coating deposited via the direct current plating process. Based on this setback, an alternative mechanism was proposed by Nielson et al. [160] more recently. According to Nielson et al. [160], though a very strong textured Cr coating is formed via the direct current method; however, the presence of fine fiber-shaped crystals of 10–20 nm perpendicular to the direction of the growth was also observed in their study. Moreover, they observed a significant amount of Cr2O3 nanocrystal inclusions growing epitaxially on the chromium matrix. Based on these additional findings, it was difficult to explain the mechanism of Cr crystallization with just a phase transformation from β-Cr to α-Cr; instead, they presented a restriction of crystal growth by inhibiting species likely to be hydrogen adatoms. In other words, they explained that the presence of microcracks in Cr coating produced was a result of tensile stress originating from grains accommodating boundary misfit at grain coalescence. Although both models have fundamental disparities, a high concentration of hydrogen adatoms is believed to be the key to crack formation in both models, and this has given clue(s) in proposing techniques/solutions for crack-free Cr coating deposition.
Retrospectively, some suggestions have been put forward towards the formation of crack-free Cr coatings, and they are summarized as follows: Firstly, some researchers proposed that crack-free Cr coatings can be obtained at modified process parameters such as a higher temperature (>70°C) and modified composition of the electrolyte bath, as mentioned earlier [144,160,161]. Their argument is from the standpoint that, above 70 °C, β-Cr formation and subsequent transformation, which leads to crack induced by residual stress, is too unstable to be formed during the high temperature plating process. Hence, crack-free α-Cr is deposited directly by the hot chromium process, as was obtained by Yeo S. et al. [161], with an increase in temperature from 50°C to 80°C (as shown in Figure 7), and Leisner P. et al. [144]. Similarly, Nielson et al. [160] reported that, as the temperature increases up to or above 7 °C, the inhibition of crystal growth decreases drastically, which results in very low stress that is insufficient in initiating cracks in the Cr deposit. The formation of a crack-free Cr coating may have been achieved with the hot chromium electroplating process; however, it has been observed to have its consequences, which are low current efficiency (usually about 10%) [144] and decrease in hardness of the coating (usually less than 800 Hv).
![Figure 7. SEM micrograph of Cr coatings on HT9 cladding material with an increase in temperature: (a) surface morphology and (b) cross-sectional views. Reprinted with permission from [161]. Copyright 2020 Elsevier. The SEM micrograph at 80°C shows no crack formed on/in the Cr coating.](/images/images/whitepapers/advancescrniplating/7.jpg) Figure 7. SEM micrograph of Cr coatings on HT9 cladding material with an increase in temperature: (a) surface morphology and (b) cross-sectional views. Reprinted with permission from [161]. Copyright 2020 Elsevier. The SEM micrograph at 80°C shows no crack formed on/in the Cr coating.
Figure 7. SEM micrograph of Cr coatings on HT9 cladding material with an increase in temperature: (a) surface morphology and (b) cross-sectional views. Reprinted with permission from [161]. Copyright 2020 Elsevier. The SEM micrograph at 80°C shows no crack formed on/in the Cr coating.
Secondly, crack-free Cr coating can be deposited under modulated current conditions (i.e., pulse current plating), as reported by several authors [144,162,163,164]. This is obtainable by applying high-frequency (>1 kHz) unipolar current pulsation [162,163,164]. This approach may have been successful in the laboratory, but it is less likely that sufficient precision can be obtained on an industrial scale, where currents of several thousand amperes are often employed. Hence, the search for a more promising way of producing crack-free Cr coating both in the laboratory and industrial space continued over the years. In 1948, a French patent proposed that the low-frequency periodic reversal (PR) electroplating method is appropriate for producing crack-free Cr coating in both the laboratory and industrial space [165]. Their findings showed the possibility of obtaining smooth coatings with lower intrinsic strain, which are more uniform in thickness and crack-free. Later on, the process was studied further by some authors [166,167,168], who confirmed that it is possible to obtain crack-free coatings at the PR process parameters that could be scaled up to industrial size. Exploring the PR plating mode further, Leisner P. et al.’s [144] investigation revealed that it is possible to decrease the intrinsic stress in chromium PR plated coatings by decreasing the cathodic charge in each cycle. This possibility was ascribed to the more frequent oxidation of inhibiting species on the surface that tends to reduce the grain boundary misfit.
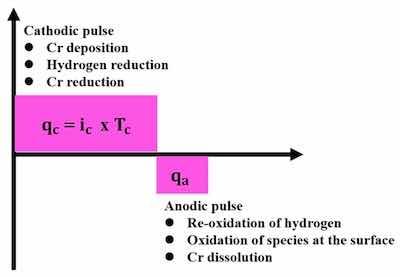 Figure 8. Principles of the pulse reversal electroplating method.Figure 8 schematically depicts the principle of the PR electroplating method, which shows that pulse reversal plating consists of bipolar pulse cycles, and the electrical charge density (q) in each pulse is a product of pulse duration (T) and current density (i), as represented for the cathode arm as ic and Tc, respectively. The PR principle in Figure 8 also depicts that anodic and cathodic charge densities (qa and qc, respectively) are important parameters in the optimization of PR plating of hard chromium.
Figure 8. Principles of the pulse reversal electroplating method.Figure 8 schematically depicts the principle of the PR electroplating method, which shows that pulse reversal plating consists of bipolar pulse cycles, and the electrical charge density (q) in each pulse is a product of pulse duration (T) and current density (i), as represented for the cathode arm as ic and Tc, respectively. The PR principle in Figure 8 also depicts that anodic and cathodic charge densities (qa and qc, respectively) are important parameters in the optimization of PR plating of hard chromium.
4.2.2. Issues of Electroplated Ni Coating
The predominant challenges with electroplated Ni coatings relate to intrinsic stress, poor adhesion/peeling off, deposit roughness, and pitting deposition. Intrinsic stress arising from hydrogen incorporation in the Ni coating layer can lead to significant yield issues, such as cracking of the coating [169,170,171,172]. Additionally, intrinsic stress can be related to poor adhesion in that it can overcome the adhesion between the substrate and the Ni coating layer if not properly managed, thereby leading to a decrease in adhesion strength and, in the worst scenario, peeling off of the Ni coating layer [148]. Other reasons such as inadequate surface preparation, poor pretreatment, and drastic change in bath chemistry, especially the operating pH, have been reported as possible reasons for adhesion failure [170,173,174]. In regard to the deposit roughness defect, Go’ral A. et al. [175] and others [176,177] reported that the presence of suspended solid(s) in solution, improper surface preparation, poor pretreatment, high pH, and high temperature, which causes an increase in bath activity, can cause deposit roughness. However, this can be resolved by Ni electrolyte bath filtration, maintaining good chemical control of the bath and proper pretreatment, as suggested by previous studies [175,177]. The use of additives such as dispersive agents or wetting agents in the plating bath has also been reported to reduce the surface tension, which leads to surface roughness [178,179].
For example, Oliveria et al. [179] fabricated a smooth and crack-free nickel coating with the addition of a 0.39 M concentration of glycerol in the Ni plating bath. Figure 9a–j depicts the SEM micrograph of Ni electrodeposits formed from Ni Wath baths in the absence and presence of different glycerol, mannitol, or sorbitol concentrations at −1.40 V and −1.60 V, respectively, and with a charge density of 2 C cm−2. Comparing the micrograph in Figure 9a–j, it can be observed that Ni films obtained in the presence of polyalcohol at −1.40 V did not present cracks, and those obtained from baths containing 0.39 M mannitol showed much smoother surfaces (Figure 9f). Pitting deposition in Ni electroplated coatings is primarily caused by the presence of contaminants such as organic contaminants and particles in the electroplating bath [180]. Moreover, Péter L. et al. [177] reported that the proper preparation of substrate material is important in depositing a pit-free Ni deposit, because if a substrate is porous or pitted, it is almost impossible for the Ni coating to fill up the void, thereby giving rise to a pitted deposit. Similarly, previous studies have revealed that the addition of additives in the electroplating bath helps to reduce the pitting in Ni electrodeposited coatings [179,180,181,182,183]. Table 1 presents a series of studies highlighting the effects of additives in Ni electrodepositions and improvements made so far in Ni coatings with such inclusion.
![Figure 9. SEM micrographs of Ni coating of 2.0 C cm−2 obtained chronoamperometrically from −0.20 to −1.40 V. Electrolytic solution: (a) Wath (W1); (b) W1 + 0.26 M glycerol, (c) W1 + 0.39 M glycerol, (d) W2 + 0.52 M glycerol, (e) W1 + 0.26 M mannitol, (f) W1 + 0.39 M mannitol, (g) W2 + 0.52 M mannitol; (h) W1 + 0.26 M sorbitol, (i) W1 + 0.39 M sorbitol, and (j) W2 + 0.52 M sorbitol. Reprinted with permission from [179].](/images/images/whitepapers/advancescrniplating/9.jpg) Figure 9. SEM micrographs of Ni coating of 2.0 C cm−2 obtained chronoamperometrically from −0.20 to −1.40 V. Electrolytic solution: (a) Wath (W1); (b) W1 + 0.26 M glycerol, (c) W1 + 0.39 M glycerol, (d) W2 + 0.52 M glycerol, (e) W1 + 0.26 M mannitol, (f) W1 + 0.39 M mannitol, (g) W2 + 0.52 M mannitol; (h) W1 + 0.26 M sorbitol, (i) W1 + 0.39 M sorbitol, and (j) W2 + 0.52 M sorbitol. Reprinted with permission from [179].
Figure 9. SEM micrographs of Ni coating of 2.0 C cm−2 obtained chronoamperometrically from −0.20 to −1.40 V. Electrolytic solution: (a) Wath (W1); (b) W1 + 0.26 M glycerol, (c) W1 + 0.39 M glycerol, (d) W2 + 0.52 M glycerol, (e) W1 + 0.26 M mannitol, (f) W1 + 0.39 M mannitol, (g) W2 + 0.52 M mannitol; (h) W1 + 0.26 M sorbitol, (i) W1 + 0.39 M sorbitol, and (j) W2 + 0.52 M sorbitol. Reprinted with permission from [179].
Table 1. A summary of previous studies showing the effects of additives on electrodeposited Ni coatings.
- Riastuti et al.: The effect of saccharin on the reduction of crystallite size and electroplated nickel properties were investigated. Their results showed that the lowest crystallite size of 35 nm was obtained at an experimental condition of 10 g/L addition of saccharin into the electrolytic bath. Also, it was found that increasing the saccharin concentration of the bath led to decreased corrosion rate of the Ni coating, and an increase in hardness and adhesive strength of the coating to the substrate. [184]
- Busi S. and Manaf A.: Investigated the influence of saccharin on the electrodeposition of Ni alloy on a flexible substrate. Based on their findings, the addition of saccharin modified the nuclei growth mechanism, making the growth phase slower than that in the saccharin-free electrolyte. The inclusion of saccharin also increased the current efficiency of Ni alloy, and slightly enhanced the deposition rate by inhibiting hydrogen evolution reaction. [185]
- Nakamura Y.: Studied the effect of aliphatic alcohols such as n-propyl alcohol, allyl alcohol, propargyl alcohol and saccharin on the surface morphology and crystal orientation of electrodeposited nickel from a Wath bath. The three different aliphatic alcohols and saccharin were adsorbed on the electrode and inhibited the reduction of nickel ions. The inhibitory effect on the reduction of nickel ion increased in the order of n-propyl alcohol, allyl alcohol and propargyl alcohol. The addition of saccharin to the electrolyte bath enabled the formation of fine-grained crystals with a relatively low surface roughness. While the Wath bath without organic additives produced a large granular electrodeposit. A finer-grained, smooth and compact nickel coating, which had a preferred orientation with a (111) plane parallel to the surface, was obtained from the Wath bath containing both saccharin and propargyl alcohol. [186]
- Abbot et al.: Examined the effect of four additives (nicotinic acid (NA), methylnicotinate (MN), 5,5-dimethylhydrantoin (DMH) and boric acid (BA) on electrolytic nickel plating. Their findings revealed that the additives show limited influence on the bulk Ni(II) speciation, however, a significant positive influence on the electrochemical behavior of Ni deposit was observed. A minute concentration (15 mM) of NA and MN showed inhibition of Ni(II) reduction, whereas a high concentration of DMH and BA are required for a modest difference in behavior from the additive-free system. NA and MN addition also significantly altered the nucleation and growth mechanism of the Ni deposit with respect to the additive-free system and those with DMH and BA. Each additive inclusion had the effect of producing brighter and flatter bulk electrodeposit with increased coating hardness. [187]
5. Application of Cr, Ni and Their Alloy Coatings
Cr and Ni, singly or in their combined (binary/multilayer) form, have been used for different applications, such as corrosion resistance coatings, tribological applications, etc. In order to highlight some of these applications, and compare some factors such as coatings deposition mode/deposition conditions, substrate, and additives used in relation to their physical characteristics, Table 2 was presented.
Table 2. A summary of previous studies showing the different applications of Ni, Cr, and their alloy coatings.
Cr/Copper Foil
- Deposition Mode/Condition: DC/Sulphate formate trivalent bath.
- Additives: Water soluble polymers
- Characterization Properties: The presence of the polymers improved the appearance of the coating and increased the current efficiency as well. Also, the corrosion resistance ability of the substrate was remarkable improved.
- Applications: Printed circuit boards.
- Reference: [129]
Cr (single and double layers)/Forged Steel (DINI 16959)
- Deposition Mode/Condition: DC/chromic acid.
- Additives: Boric acid as buffer
- Characterization Properties: The tribology properties of single hard, single crack-free and duplex (hard layer on top of the crack-free sublayer) Cr coatings on the forged steel was studied for fixed thickness. The single hard Cr coating had the best wear resistance, while the single crack-free coating has the worst wear resistance.
- Applications: Tribology application.
- Reference: [69]
Ni/Copper Substrate
- Deposition Mode/Condition: Pulse plating/Ni sulphate bath, and pulse frequency range of 1–100 Hz.
- Additives: Boric acid
- Characterization Properties: The crystallite size of the deposit increased with increase in current density and duty cycle. Ni coatings with (200) texture are more ductile, while Ni coating with (111) texture had an improved microhardness.
- Applications: Ni-plated copper is ideal for application with high thermal demand, such as wire and cables, integrated circuits, conductors, etc.
- Reference: [57]
Ni-Cr/Copper Substrate
- Deposition Mode/Condition: DC/plating time (5–25 min) and current density (2–8 A/dm2) were varied to study their effects on the coating porosity.
- Additives: Boric acid
- Characterization Properties: A porous coating with very small grain particles of Ni and Cr were produced. At low current density and higher plating time, small sized pores that are large in number were uniformly distributed. While the vice versa was observed at high current density and low plating time.
- Applications: The porous nature of the alloy coating enables it to find application in the field of filler membrane and electrolytic purification.
- Reference: [188]
Ni Strike-Ni-Cr/Alloy Carbon Steel
- Deposition Mode/Condition: DC and PC/current density of 55 A/dm2 and electrolyte temperature of 55 °C for the Cr coating, and 10 A/dm2 and 55 °C for the Ni coating.
- Additives: Boric acid
- Characterization Properties: The wear resistance properties of the Cr coating layer were enhanced by using nickel middle layer.
- Applications: Where high hardness and high wear resistance materials are required, such as bolt and crankshaft in the automobile industry.
- Reference: [189]
Ni/Copper Plate
- Deposition Mode/Condition: DC/PC/PRC
- Additives: Undisclosed additive to control deposit structure and properties.
- Characterization Properties: A decrease in grain size and a greater level of uniformity of the deposit were obtained by using the PC and PCR techniques. Additionally, all coatings produced at different conditions improved the corrosion resistant property of the copper substrate.
- Applications: Anti-corrosion applications in production lines
- Reference: [116]
Cr, Ni, and Cr-Ni Binary Alloy/Low Carbon Steel Sheet
- Deposition Mode/Condition: PC/modulated agitation.
- Additives: Sodium dodecyl sulphate surfactant
- Characterization Properties: Cr and Ni single layers have lower corrosion resistance than the Cr-Ni alloy coating owing to the presence of surface cracks and pores in the former.
- Applications: Railway lines and structural engineering plates.
- Reference: [190]
In order to meet several other applications demands, some authors combined Ni and/or Cr with other elements of the target interest to produce binary or multilayer coatings. Recently, this kind of study is gaining interest, and as such, a summary of different binary/multilayer coatings via electrodeposition, their applications, and comparison is presented in Table 3.
Table 3. A summary of previous studies showing different binary alloys of Cr and/or Ni coatings and their comparisons.
Cr and Ni/Cr Alloy Coatings On Alloy Carbon Steel
- Technique: Electrodeposition, DC and PC.
- Remarks: The Ni/Cr alloy coating gave a better hardness and wear resistance than the single Cr layered coating.
- Applications: Bolts and crankshaft in the automobile industry, i.e., where high hardness and high wear resistance are required.
- Reference: [189]
Ni and Ni-Co Coatings on Brass
- Technique: Electrodeposition, DC.
- Remarks: Ni-Co alloy coating in the proportion of Ni42Co58 gave a better hardness, roughness and conductivity close to the coins in circulation than pure Ni coating.
- Applications: Suitable material for coin production.
- Reference: [76]
Ni-P and Ni-Mo Coatings on Electromagnetic Iron Rails
- Technique: Electrodeposition, DC.
- Remarks: Their finding showed that the electrically ablated surface areas of the rails both coated and uncoated are in the following order based on the effectiveness of the coatings: annealed Ni-Mo < Ni-Mo < annealed Ni-P < Ni-P < iron material. This depicts that the annealed Ni-Mo coating has the best electrical ablation wear resistance.
- Applications: These types of coatings are aimed at reducing the electrical ablation of electromagnetic iron rails, which seriously affects the service live performance of the electromagnetic catapult system.
- Reference: [191]
Cr-C, Ni-P and Ni-B Coatings on 4140 Alloy Steel
- Technique: Electrodeposition, DC.
- Remarks: The findings of this study are as follows: wear rate: Ni-B < Ni-P < Cr-C; hardness and adhesion strength: Ni-P < Cr-C < Ni-B; Corrosion resistance: Cr-C < Ni-P < Ni-B. These findings connote that the Ni-B coating is most suitable for protecting the alloy steel from corrosion and wear damage.
- Applications: Industrial applications such as blades, gears etc., where high wear resistance and corrosion resistance are required.
- Reference: [192]
6. Prospects of Cr and Ni Electroplating
The future of research on Cr and Ni electroplating lies in the following direction:
(1) Enhancing the understanding of the basic aspect of Cr and Ni electrodeposition and bath chemistry: The use of additives and their effects have been promising in Cr and Ni electroplating; however, it is important to ensure that those that are applied in industrial applications are as harmless as possible. Consequently, more investigations still need to be performed towards making Cr and Ni electrodeposition eco-friendlier. For instance, the use of organic and green stabilizers, which are still new to these processes, require furthers investigation and modification.
(2) Tailored and Patterned Structures: Many modern materials such as semiconductors, electrocatalysts, and magnetic materials demand fine control of the dopant levels together with tailored morphology, nanostructure, and elemental/phase compositions. Tailoring and patterning novel structures using modified, intelligent, and targeted deposition conditions in Cr and Ni electrodepositions can be useful for these purposes. Furthermore, incorporating nanoparticles into Cr and Ni deposits could lead to producing coatings with specific characteristics, such as premium wear resistance, good stress corrosion cracking resistance, acceptable thermal stability, and high hardness. Thus, developing strategies for improving the number of nanoparticles incorporated into electrodeposited Cr and Ni coatings, such as the development of horizontal electrodes, can be supposed as a future trend for investigation.
(3) Thermal stability and high temperature applications: More recently, Cr and Ni/Ni alloys electrodeposited coatings are being tried for high temperature applications owing to their high thermal stabilities. Industries such as nuclear power plants and refineries are sectors that are beginning to benefit from Cr and Ni-modified coatings. Comprehensively, assessments have shown that metallic Cr coating is the most promising technology for further development in tackling the problem of modular corrosion in a boiling water reactor, because only chromia is stable at these high temperatures and dissolved oxygen conditions [193]. Similarly, Cr coating is more suitable for a light water reactors coolant environment where high corrosion resistance, neutron irradiation stability, and oxidation resistance to high temperature steam are demanded. Thus, accident-tolerant fuel materials could benefit from Cr coating applications.
(4) Industrialization of Cr and Ni electroplating: The industrialization of the Cr and Ni electroplating processes has been and is still a big challenge. Very few studies have focused on the replenishment, recycling of baths, and retreatment to facilitate their implementation in industries, even when they are utterly essential to ensure the viability and sustainability of the technology. These kinds of studies will also make it possible to fully determine the implication of applying Cr and Ni electroplated coatings from both an environment and economic perspective, making use of methods such as a life cycle assessment, which is difficult as long as the aging behavior of Cr and Ni plating baths are not fully characterized.
(5) Substrate surface condition: Surface condition and pretreatment of the substrate has become increasingly essential in early nucleation and growth in order to achieve thinner coatings, multiple but well-defined successive layers, and faster layer deposition. The surface condition of a substrate is of utmost importance for materials such as stainless steel in electrodeposition because of its fast rate im forming protective oxides; hence, proper pretreatment is needed to obtain good adhesion and quality Cr and Ni coatings on its surface. The continuous investigation and modification of surface pretreatment techniques are therefore very important.
7. Concluding Remarks
In this review, we discussed a wide range of issues pertaining to Cr and Ni electrodepositions, which are summarized in the following points:
Early studies were often motivated by the desire to improve the corrosion resistance, wear resistance, and mechanical properties of materials used in diverse applications; modern endeavors continue to improve on the past motives; however, heightened research effort has gone into the development of Cr and Ni coatings for new and improved applications such as magnetic material purposes, membrane technology, electronic devices, and semiconductors.
The understanding of the mechanism of Cr and Ni electrodeposition is very important in modifying the coatings and resolving the issues/difficulties experienced in the processing and production. This review reports that the conventional mechanism of Cr electrodeposition from a trivalent Cr bath involves two basic consecutive steps, which are electrochemical reduction of Cr3+ species to Cr2+ and then from Cr2+ species to Cr (s), and of which the rate of deposition is controlled by the diffusion of the complex Cr3+ ions to the cathode surface, while the Ni electrodeposition mechanism involves two consecutive one-electron charge transfers and the participation of an anion in the formation of an adsorbed complex.
The influence of the process parameters, such as current density, temperature, pH, type of current, and additives on the electrodeposition process and product, were discussed in detail in this review, with the aim of providing basic knowledge and ideas on the parameters necessary for targeted purposes.
Issues experienced in the electroplating processes and products of Cr and Ni electrodeposit were discussed extensively. Similarly, potential solutions via research and development were also identified and compiled in order to aid researchers in surmounting some of these challenges.
Written by Bright O. Okonkwo, Chaewon Jeong, and Changheui Jang; Department of Nuclear and Quantum Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Korea
Author Contributions: B.O.O.: Conceptualization, Writing—original, Draft preparation, Visualization, and Reviewing and Editing. C.J. (Chaewon Jeong): Review Writing—original, and Draft preparation. C.J. (Changheui Jang): Supervision, Writing–Reviewing and Editing, and Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding: This work was funded by the Nuclear R & D Program (No. 2019M2D2A2050927) of MSIT/NRF of the Republic of Korea.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Khadem, M.; Penkov, O.V.; Yang, H.K.; Kim, D.E. Tribology of multilayer coatings for wear reduction: A review. Friction 2017, 5, 248–262.
- Beltowska-Lehman, E.; Indyka, P.; Bigos, A.; Szczerba, M.J.; Guspiel, J.; Koscielny, H. Effect of current density on properties of Ni-W nanocomposite coatings reinforced with zirconia particles. Mater. Chem. Phys. 2016, 173, 524–533.
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A review. Sci. Technol. Adv. Mater. 2008, 9, 1–11.
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009–1012.
- Larson, C.; Smith, J.R. Recent trends in metal alloy electrolytic and electroless plating research: A review. Trans. Inst. Met. Finish. 2011, 89, 333–341.
- Brenner, A. Electrodeposition of Alloys; Academic Press Inc.: New York, NY, USA, 1963; Volume 1, p. 258.
- Tabakovic, I.; Qiu, M.; Riemer, S.; Sun, M.; Vas’ko, V.; Kief, M. CoFeRh alloys: Part 1. Electrodeposition of Rh and nonmagnetic CoFeRh alloy. Electrochim. Acta 2008, 53, 2483–2493.
- Tabakovic, I.; Riemer, S.; Vas’ko, V.; Kief, M. CoFeRh alloys: Part 2. Electrodeposition of CoFeRh alloys with high saturation magnetic flux density and high corrosion resistance. Electrochim. Acta 2008, 53, 8008–8014.
- Tabakovic, I.; Inturi, V.; Thurn, J.; Kief, M. Properties of Ni1−xFex (0.1 < x < 0.9) and Invar (x = 0.64) alloys obtained by electrodeposition. Electrochim. Acta 2010, 55, 6749–6754.
- Zhu, W.; Wang, S.L. Residual Stresses and Nanoindentation Testing of Films and Coatings. Rare Met. Mater. Eng. 2008, 37, 891–895.
- Li, Y.J.; Zhu, C.Z.; Wang, C.M. Controllable synthesis, characterization and microwave absorption properties of magnetic Ni1− xCoxP alloy nanoparticles attached on carbon nanotubes. J. Phys. D Appl. Phys. 2008, 41, 125303.
- Cojocaru, P.; Spreafico, M.; Gomez, E.; Valles, E.; Magagnin, L. Electrocodeposition of CoNi/barium ferrite using a forced flow cell. Surf. Coat. Technol. 2010, 205, 195–199.
- Cojocaru, P.; Magagnin, L.; Gomez, E.; Valles, E. Electrodeposition of CoNiP films with modulated magnetic behavior. Trans. Inst. Met. Fin. 2011, 89, 194–197.
- Rhen, F.M.F.; Roy, S. Electrodeposited CoNiFeP Soft-Magnetic Films for High-Frequency Applications. IEEE Trans. Magn. 2008, 44, 3917–3920.
- Ohgai, T.; Hjort, K.; Spohr, R.; Neumann, R. Electrodeposition of cobalt based ferro-magnetic metal nanowires in polycarbonate films with cylindrical nanochannels fabricated by heavy-ion-track etching. J. Appl. Electrochem. 2008, 38, 713–719.
- Zheng, Z.X.; Wang, R.; Wang, C.M. Electroless plating of Co–Zn–P thin film onto nano-diamond cores. Curr. Appl. Phys. 2011, 11, 227–230.
- Subramanian, B.; Govindan, K.; Swaminathan, V.; Jayachandran, M. Materials properties of electrodeposited NiFe and NiCoFe coatings. Trans. Inst. Met. Fin. 2009, 87, 325–329.
- Cavallotti, P.L.; Bestetti, M.; Franz, S.; Vicenzo, Z. A Hothersall Memorial Award Lecture: Nano-electrodeposition for hard magnetic layers. Trans. Inst. Met. Fin. 2010, 88, 28–34.
- Pang, J.F.; Li, Q.; Wang, W.; Xu, X.T.; Zhai, J.P. Preparation and characterization of electroless Ni–Co–P ternary alloy on fly ash cenospheres. Surf. Coat. Technol. 2011, 205, 4237–4242.
- Tai, C.Y.; Chang, J.L.; Lee, J.F.; Chan, T.S.; Zen, J.M. Preparation and characterization of an AuCu3 alloy electrode for electrocatalytic applications. Electrochim. Acta 2011, 56, 3115–3121.
- Saejeng, Y.; Tantavichet, N. Preparation of Pt–Co alloy catalysts by electrodeposition for oxygen reduction in PEMFC. J. Appl. Electrochem. 2009, 39, 123–134.
- Wang, L.; Bao, S.G.; Yi, J.H.; He, F.; Mi, Z.T. Preparation and properties of Pd/Ag composite membrane for direct synthesis of hydrogen peroxide from hydrogen and oxygen. Appl. Catal. B Environ. 2008, 79, 157–162.
- Bhandari, R.; Ma, Y.H. Pd–Ag membrane synthesis: The electroless and electro-plating conditions and their effect on the deposit’s morphology. J. Membr. Sci. 2009, 34, 50–63.
- Foletto, E.L.; da Silveira, J.V.W.; Jahn, S.L. Preparation of palladium-silver alloy membranes for hydrogen permeation. Latin Am. Appl. Res. 2008, 38, 79–84.
- Yuan, L.X.; Goldbach, A.; Xu, H.Y. Real-time monitoring of metal deposition and segregation phenomena during preparation of Pd-Cu membranes. J. Membr. Sci. 2008, 322, 39–45.
- Li, H.L.; Wang, S.M.; Jiang, L.I.; Zhang, L.D.; Liu, X.P.; Li, Z.N. Preparation and research on poisoning resistant Zr-Co based hydrogen storage alloys. Rare Met. 2008, 27, 367–370.
- Tarditi, A.M.; Cornaglia, L.M. Novel PdAgCu ternary alloy as promising materials for hydrogen separation membranes: Synthesis and characterization. Surf. Sci. 2011, 605, 62–71.
- Tarditi, A.M.; Braun, F.; Cornaglia, L.M. Novel PdAgCu ternary alloy: Hydrogen permeation and surface properties. Appl. Surf. Sci. 2011, 257, 6626–6635.
- Zhang, X.L.; Xie, X.F.; Huang, Y. Pure Ni and Pd-Ni alloy membranes prepared by electroless plating for hydrogen separation. Adv. Mater. Res. 2011, 179, 1309–1313.
- Tabuchi, T.; Hochgatterer, N.; Ogumi, Z.; Winter, M. Ternary Sn–Sb–Co alloy film as new negative electrode for lithium-ion cells. J. Power Sources 2009, 188, 552–557.
- Xue, L.G.; Fu, Z.H.; Yao, Y.; Huang, T.; Yu, A.S. Three-dimensional porous Sn–Cu alloy anode for lithium-ion batteries. Electrochim. Acta 2010, 55, 7310–7314.
- Duhin, A.; Sverdlov, Y.; Feldman, Y.; Shacham-Diamand, Y. Electroless deposition of NiWB alloy on p-type Si (1 0 0) for NiSi contact metallization. Electrochim. Acta 2009, 54, 6036–6041.
- Murase, K.; Ito, A.; Ichii, T.; Sugimura, H. Preparation of Cu-Sn layers on polymer substrate by reduction-diffusion method using ionic liquid baths. J. Electrochem. Soc. 2011, 158, D335–D341.
- Inoue, K.U.; Matsui, K.; Watanabe, M.; Honma, H. Effect of UV irradiation on electroless Cu–Ni–P plating on cycloolefin polymer. Trans. Inst. Met. Fin. 2009, 87, 51–54.
- Zeng, L.C.; Wen, W.; Li, D. Electrodeposition of coated CoZnP on nano-diamond. Electroplat. Finish. 2009, 28, 20–22.
- Duan, D.L.; Li, S.; Ding, X.J.; Jiang, S.L. Preparation of Ni–Cr alloy foams by electrodeposition technique. Mater. Sci. Technol. 2008, 24, 461–466.
- Tarozaite, R.; Sukackiene, Z.; Sudavicius, A.; Juskenas, R.; Selskis, A.; Jagminiene, A.; Norkus, E. Application of glycine containing solutions for electroless deposition of Co–P and Co–W–P films and their behavior as barrier layers. Mater. Chem. Phys. 2009, 117, 117–124.
- Hamid, Z.A.; Aal, A.A.; Shaaban, A.; Hassan, H.B. Electrodeposition of CoMoP thin film as diffusion barrier layer for ULSI applications. Surf. Coat. Technol. 2009, 203, 3692–3700.
- Aal, A.A.; Barakat, H.; Hamid, Z.A. Synthesis and characterization of electroless deposited Co–W–P thin films as diffusion barrier layer. Surf. Coat. Technol. 2008, 202, 4591–4597.
- Schlossmacher, P.; Fath, A. The effect of different additives on the electrodeposition of Ni–Fe alloys of 10–36%Fe for microsystem technology applications. J. Electrochem. Plat. Technol. 2010, 1, 20–32.
- Liu, Y.; Huang, M.L. Outgassing of materials used for thin film vacuum packages. In Proceedings of the Electronic Packaging Technology and High-Density Packaging, Beijing, China, 10–13 August 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 802–805.
- Afzali, A.; Mottaghitalab, V.; Motlagh, M.S.; Haghi, A.K. The electroless plating of Cu-Ni-P alloy onto cotton fabrics. Korean J. Chem. Eng. 2010, 27, 1145–1149.
- Sonehara, M.; Noguchi, S.; Kurashina, T.; Sato, T.; Yamasawa, K.; Miura, Y. Development of an Electromagnetic Wave Shielding Textile by Electroless Ni-Based Alloy Plating. IEEE Trans. Magn. 2009, 45, 4173–4175.
- Pewnim, N.; Roy, S. Effect of fluorosurfactant on copper–tin reduction from methanesulphonic acid electrolyte. Trans. Inst. Met. Fin. 2011, 89, 206–209.
- Lee, Y.G.; Park, J.G.; Lee, C.W.; Jung, J.P. Electrodeposition of the Sn-58 wt.%Bi layer for low-temperature soldering. Met. Mater. Int. 2011, 17, 117–121.
- Tsai, Y.D.; Hu, C.C. Composition Control of Sn–Bi Deposits: Interactive Effects of Citric Acid, Ethylenediaminetetraacetic Acid, and Poly(ethylene glycol). J. Electrochem. Soc. 2009, 156, D490–D496.
- Dimitrovska, A.; Kovacevic, R. The Effect of Micro-Alloying of Sn Plating on Mitigation of Sn Whisker Growth. J. Electron. Mater. 2009, 38, 2726–2734.
- Sarobol, P.; Pedigo, A.; Blendell, J.; Handwerker, C.; Su, P.; Li, L.; Xue, J. A Synchrotron Micro-Diffraction Investigation of Crystallographic Texture of High-Sn Alloy Films and Its Effects on Whisker Growth. In Proceedings of the 60th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 1–4 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 162–169.
- Tang, P.T.; Jaskula, M.; Kubiczek, M.; Mizushima, I.; Pantleon, K.; Arentoft, M. Pulse reversal plating of nickel–cobalt alloys. Trans. Inst. Met. Fin. 2009, 87, 72–77.
- Yang, J.M.; Zhu, D.; Kim, D.H. Influence of high frequency pulse current on properties of electroformed nanocrystalline Ni–Mn alloys. Trans. Inst. Met. Fin. 2008, 86, 98–102.
- Hsieh, Y.C.; Lu, Y.J.; Wu, P.W.; Chang, Y.M.; Chiu, Y.F. Preparation of nanoparticulate PtRu (Pt65Ru35) electrocatalyst for direct methanol fuel cells. In Proceedings of the 8th Symposium on Proton Exchange Membrane Fuel Cells, Honolulu, HI, USA, 12–17 October 2008; Electrochemical Society: Piscataway, NJ, USA, 2008; pp. 473–481.
- Hosseini, M.; Ebrahimi, S. The effect of Tl(I) on the hard gold alloy electrodeposition of Au–Co from acid baths. J. Electroanal. Chem. 2010, 645, 109–114.
- Han, Q.; Cui, S.A.; Pu, N.W.; Chen, J.S.; Liu, K.R.; Wei, X.J. A study on pulse plating amorphous Ni–Mo alloy coating used as HER cathode in alkaline medium. Int. J. Hydrog. Energy 2010, 35, 5194–5201.
- Beltowska-Lehman, E.; Indyka, P. Kinetics of Ni-Mo electrodeposition from Ni-rich citrate baths. Thin Solid Films 2012, 520, 2046–2051.
- Tsyntsaru, N.; Cesiulis, H.; Pellicer, E.; Celis, J.-P.; Sort, J. Structural, magnetic, and mechanical properties of electrodeposited cobalt-tungsten alloys: Intrinsic and extrinsic interdependencies. Electrochim. Acta 2013, 104, 94.e103.
- Aliofkhazraei, M.; Walsh, F.C.; Zangari, G.; Oçkar, H.K.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Appl. Surf. Sci. Adv. 2021, 6, 100141.
- Sajjadnejad, M.; Omidvar, H.; Javanbakht, M.; Mozafari, A. Characterization of pure nickel coatings fabricated under pulse current condition. Int. J. Mater. Met. Eng. 2015, 9, 1061–1065.
- Zeng, Z.; Wang, L.; Liang, A.; Zhang, J. Tribological and electrochemical behavior of thick Cr–C alloy coatings electrodeposited in trivalent chromium bath as an alternative to conventional Cr coatings. Electrochim. Acta 2006, 52, 1366–1373.
- Baral, A.; Engelken, R. Modeling, optimization, and comparative analysis of trivalent chromium electrodeposition from aqueous glycine and formic ccid baths. J. Electrochem. Soc. 2005, 152, C504.
- Chang, J.H.; Hsu, F.Y.; Liao, M.J.; Huang, C.A. A study of direct and pulse-current chromium electroplating on rotating cylinder electrode (RCE). Appl. Surf. Sci. 2007, 253, 6829.
- Huang, C.A.; Lin, W.; Liao, M.J. The electrochemical behaviour of the bright chromium deposits plated with direct- and pulse-current in 1 M H2SO4. Corros. Sci. 2006, 48, 460–472.
- Lausmann, G.A. Electrolytically deposited hard chrome. Surf. Coat. Technol. 1996, 86–87, 814–823.
- Song, Y.B.; Chin, D.T. Current efficiency and polarization behavior of trivalent chromium electrodeposition process. Electrochim. Acta 2002, 48, 349–356.
- Edigaryan, A.A.; Safonov, V.A.; Lubnin, E.N.; Vykhodtseva, L.N.; Chusova, G.E.; Polukarov, Y.M. Properties and preparation of amorphous chromium carbide electroplates. Electrochim. Acta 2002, 47, 2775–2786.
- Chien, C.W.; Liu, C.L.; Chen, F.J.; Lin, K.H.; Lin, C.S. Microstructure and properties of carbon–sulfur-containing chromium deposits electroplated in trivalent chromium baths with thiosalicylic acid. Electrochim. Acta 2012, 72, 74–80.
- Safonov, V.A.; Vykhodtseva, L.N.; Polukarov, Y.M.; Safonova, O.V.; Smolentsev, G.; Sikora, M.; Eeckhout, S.G.; Glatzel, P. Valence-to-Core X-ray Emission spectroscopy identification of carbide compounds in nanocrystalline Cr coatings deposited from Cr (III) Electrolytes Containing Organic Substances. J. Phys. Chem. B 2006, 110, 23192–23196. [PubMed]
- Eeckhout, S.G.; Safonova, O.V.; Smolentsev, G.; Biasioli, M.; Safonov, V.A.; Vykhodtseva, L.N.; Sikora, M.; Glatzel, P. Cr local environment by valence-to-core X-ray emission spectroscopy. J. Anal. At. Spectrom. 2009, 24, 215–223.
- Safonova, O.V.; Vykhodtseva, L.N.; Polyakov, N.A.; Swarbrick, J.C.; Sikora, M.; Glatzel, P.; Safonov, V.A. Chemical composition and structural transformations of amorphous chromium coatings electrodeposited from Cr (III) electrolytes. Electrochim. Acta 2010, 56, 145–153.
- Sohi, M.H.; Kashi, A.A.; Hadavi, S.M.M. Comparative tribological study of hard and crack-free electrodeposited chromium coatings. J. Mater. Process. Technol. 2003, 138, 219–222.
- Huang, C.A.; Liu, Y.W.; Yu, C.; Yang, C.C. Role of carbon in the chromium deposit electroplated from a trivalent chromium-based bath. Surf. Coat. Technol. 2011, 205, 3461–3466.
- Danilov, F.I.; Protsenko, V.S.; Gordiienko, V.O.; Kwon, S.C.; Lee, J.Y.; Kim, M. Nanocrystalline hard chromium electrodeposition from trivalent chromium bath containing carbamide and formic acid: Structure, composition, electrochemical corrosion behavior, hardness and wear characteristics of deposits. Appl. Surf. Sci. 2011, 257, 8048–8053.
- Nam, K.S.; Lee, K.H.; Kwon, S.C.; Lee, D.Y.; Song, Y.S. Improved wear and corrosion resistance of chromium (III) plating by oxynitrocarburising and steam oxidation. Mater. Lett. 2004, 58, 3540–3544.
- Zeng, Z.; Zhang, J. Correlation between the structure and wear behavior of chromium coatings electrodeposited from trivalent chromium baths. Tribol. Lett. 2008, 30, 107–111.
- Lee, C.-Y.; Mao, W.-T.; Ger, M.-D.; Lee, H.-B. A study on the corrosion and wear behavior of nanocrystalline Ni-Mo electrodeposited coatings. Surf. Coat. Technol. 2019, 366, 286–295.
- Jinlong, L.; Zhuqing, L.; Tongxiang, L.; Suzuki, K.; Hideo, M. Effect of tungsten on microstructures of annealed electrodeposited Ni-W alloy and its corrosion resistance. Surf. Coat. Technol. 2018, 337, 516–524.
- Schweckandt, D.S.; Aguirre, M.C. Electrodeposition of Ni-Co alloys. Determination of properties to be used as coins. Procedia Mater. Sci. 2015, 8, 91–100.
- Pirota, K.R.; Vazquez, M. Arrays of electroplated multilayered Co/Cu nanowires with controlled magnetic anisotropy. Adv. Eng. Mater. 2005, 7, 1111–1113.
- Rahman, A.Z.M.S.; Chia, P.Y.; Haseeb, A. Nanomechanical properties of intermetallic compounds formed in electrodeposited Cu/Sn and Cu/Ni/Sn multilayer interconnects. In Proceedings of the 36th International Electronics Manufacturing Technology Conference, Johor Bahru, Malaysia, 11–13 November 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–4.
- Omar, I.M.A.; Emran, K.M.; Aziz, M. Electrodeposition of Ni-Co Film: A Review. Int. J. Electrochem. Sci. 2021, 16, 150962.
- Al Raddadi, R.M. Cathodic Codeposition of Nickel-Cobalt Alloy Coatings from Acidic Glycine Complex Baths. Master’s Thesis, Taibah University, KSA, Medina, Saudi Arabia, 2014.
- Zhu, Y.L.; Katayama, Y.; Miura, T. Effects of acetonitrile on electrodeposition of Ni from a hydrophobic ionic liquid. Electrochim. Acta 2010, 55, 9019–9023.
- Cârâc, G.; Ispas, A. Effect of nano-Al2O3 particles and of the Co concentration on the corrosion behavior of electrodeposited Ni–Co alloys. J. Solid State Electrochem. 2012, 16, 3457–3465.
- Ghaziof, S.; Golozar, M.A.; Raeissi, K. Characterization of as-deposited and annealed Cr–C alloy coatings produced from a trivalent chromium bath. J. Alloys Compd. 2010, 496, 164–168.
- White, H.; Twardoch, U.M. The chemistry and electrochemistry associated with the electroplating of group VIA transition metals. J. Appl. Electrochem. 1987, 17, 225–242.
- Orinakova, R.; Turonova, A.; Kladekova, D.; Galova, M.; Smith, R.M. Recent developments in the electrodeposition of nickel and some nickel-based alloys. J. Appl. Electrochem. 2006, 36, 957–972.
- Khani, H.; Brennecke, J.F. Hard chromium composite electroplating on high-strength stainless steel from a Cr(III)-ionic liquid solution. Electrochem. Comm. 2019, 107, 106537.
- Xu, L.; Pi, L.; Dou, Y.; Cui, Y.; Mao, X.; Lin, A.; Fernandez, C.; Peng, C. Electroplating of thick hard chromium coating from a trivalent chromium bath containing a ternary complexing agent: A methodological and mechanistic study. ACS Sustain. Chem. Eng. 2020, 41, 15540–15549.
- Phuong, N.V.; Kwon, S.C.; Lee, J.Y.; Lee, S.J.; Huy, B.T.; Lee, Y.-I. Mechanistic study on the effect of polyethylene glycol molecule in a trivalent chromium electrodeposition process. Microchem. J. 2011, 99, 7–14.
- Yao, Y.; Wei, Q.; Sun, M.; Chen, Y.; Ren, X. Environmentally friendly chromium electrodeposition: Effect of pre-electrolysis on a Cr(III) bath in an anion-exchange membrane reactor. RSC Adv. 2013, 3, 13131–13136.
- Wu, W.; Huang, J.; Nather, J.; Amanina, N.; Omar, B.; Koster, F.; Lampke, T.; Liu, Y.; Pan, H.; Zhang, Y. Texture orientation, morphology and performance of nanocrystalline nickel coatings electrodeposited from a Watts-type bath: Effects of H3BO3 concentration and plating time. Surf. Coat. Technol. 2021, 424, 127648.
- Burstein, G.T.; Chin, X.Y.; Haslam, G.E.; Ren, G.; Sonto, R.M.; Speckert, L. Proceedings of the 18th International Corrosion Congress Plenary, Perth, Australia, 20–24 November 2011.
- Allongue, P.; Cagnon, L.; Gomez, C.; Gundel, A.; Costa, V. Electrodeposition of Co and Ni/Au(111) ultrathin layers. Part I: Nucleation and growth mechanisms from in situ STM. Surf. Sci. 2004, 557, 41.
- Supicová, M.; Rozik, R.; Trnková, L.; Oriňáková, R.; Gálová, M. Influence of boric acid on the electrochemical deposition of Ni. J. Solid State Electrochem. 2006, 10, 61–68.
- Gomez, E.; Pollina, R.; Valles, E. Nickel electrodeposition on different metallic substrates. J. Electroanal. Chem. 1995, 386, 45–56.
- Güler, E.S. Effects of Electroplating Characteristics on the Coating Properties. Electrodepos. Compos. Mater. 2016, 1, 27–37.
- Ebrahimi, F.; Ahmed, Z. The effect of current density on properties of electrodeposited nanocrystalline nickel. J. Appl. Electrochem. 2003, 33, 733–739.
- Rashidi, A.M.; Amadeh, A. The effect of current density on the grain size of electrodeposited nanocrystalline nickel coatings. Surf. Coat. Technol. 2008, 202, 3772–3776.
- Uhm, Y.R.; Park, K.Y.; Choi, S.J. The effects of current density and saccharin addition on the grain size of electroplated nickel. Res. Chem. Intermed. 2015, 41, 4141–4149.
- Bayramoglu, M.; Onat, B.; Geren, N. Statistical optimization of process parameters to obtain maximum thickness and brightness in chromium plating. J. Mater. Process. Technol. 2008, 203, 277–286.
- Gómez, E.; Pollina, R.; Vallés, E. Morphology and structure of nickel nuclei as a function of the conditions of electrodeposition. J. Electroanal. Chem. 1995, 397, 111–118.
- Jinlong, L.; Tongxiang, L.; Chen, W. Effect of electrodeposition temperature on grain orientation and corrosion resistance of nanocrystalline pure nickel. J. Solid State Chem. 2016, 240, 109–114.
- Lin, C.S.; Peng, K.C.; Hsu, P.C.; Chang, L.; Chen, C.H. Effect of bath temperature on microstructure of sulfamate nickel electrodeposits. Mater. Trans. JIM 2000, 41, 777–782.
- Susetyo, F.B.; Fajrah, M.C.; Soegijono, B. Effect of electrolyte temperature on properties of nickel film coated onto copper alloy fabricated by electroplating. E-J. Surf. Sci. Nanotechnol. 2020, 18, 223–230.
- Mardanifar, A.; Mohseni, A.; Mahdavi, S. Wear and corrosion of Co-Cr coatings electrodeposited from a trivalent chromium solution: Effect of heat treatment temperature. Surf. Coat. Technol. 2021, 422, 127535.
- Behbahani, M.; Najafisayar, P.; Pakshir, M. A new method for electroplating of rack-Free chromium coatings. Iran. J. Chem. Chem. Eng. 2018, 37, 117–127.
- Mersagh-Dezfuli, S.; Sabzi, M. Deposition of ceramic nanocomposite coatings by electroplating process: A Review of layer-deposition mechanisms and effective parameters on the formation of the coating. Ceram. Int. 2019, 45, 21835–21842.
- Mandich, N.V.; Fellow, A. pH, hydrogen evolution and their significance in electroplating operations. Tech. Artic. 1999, 89, 2–5.
- Haque, M.; Hoque, M.; Islam, M.; Mustafa, C. Effect of various operating parameters on trivalent chromium electroplating. J. Sci. Res. Rep. 2017, 13, 1–9.
- Protsenko, V.S.; Danilov, F.I.; Gordiienko, V.O.; Kwon, S.C.; Kim, M.; Lee, J.Y. Electrodeposition of hard nanocrystalline chrome from aqueous sulfate trivalent chromium bath. Thin Solid Film. 2011, 520, 380–383.
- Protsenko, V.S.; Gordiienko, V.O.; Danilov, F.I.; Kwon, S.C. Preparation and characterization of nanocrystalline hard chromium coatings using eco-friendly trivalent chromium bath. E-J. Chem. 2011, 8, 1925–1929.
- Nasirpouri, F.; Sanaeian, M.R.; Samardak, A.S.; Sukovatitsina, E.V.; Ognev, A.V.; Chebotkevich, L.A.; Hosseini, M.G.; Abdolmaleki, M. An investigation on the effect of surface morphology and crystalline texture on orrosion behavior, structural and magnetic properties of electrodeposited nanocrystalline nickel films. Appl. Surf. Sci. 2014, 292, 795–805.
- Borkar, T.; Harimkar, S.P. Effect of electrodeposition conditions and reinforcement content on microstructure and tribological properties of nickel composite coatings. Surf. Coat. Technol. 2011, 205, 4124–4134.
- Shao, L.; Du, L.; Wang, L. Development of nanocrystalline nickel by pulse reverse microelectroforming. Micro Nano Lett. 2010, 5, 165–170.
- Tang, P.T.; Leisner, P.; Møller, P. Improvements of nickel deposit characteristics by pulse plating. In SUR/FIN Manufacturing Technol. Conference; AESF Society: Orlando, FL, USA, 1993.
- Chung, C.K.; Chang, W.T. Effect of pulse frequency on the morphology and nanoindentation property of electroplated nickel films. Microsyst. Technol. 2007, 13, 537–541.
- Aperador Chaparro, W.A.; Lopez, E.V. Electrodeposition of nickel plates on copper substrates using PC and PRC. Matéria 2007, 12, 583–588.
- Pavlatou, E.A.; Raptakis, M.; Spyrellis, N. Synergistic effect of 2-Butyne-1,4-Diol and pulse plating on the structure and properties of nickel nanocrystalline deposits. Surf. Coat. Technol. 2007, 201, 4571–4577.
- Beyragh, M.S.; Asl, S.K.; Norouzi, S. A Comparative research on corrosion Behavior of a standard, crack-free and duplex hard chromium coatings. Surf. Coat. Technol. 2010, 205, 2605–2610.
- Huang, C.A.; Chen, J.Y.; Leu U., W. Corrosion behavior of as-plated and annealed Cr-C coatings deposited on steel. Key Eng. Mater. 2017, 724, 12–15.
- Yeo, S.; Kim, J.H.; Yun, H.S. Effect of pulse current and coating thickness on the microstructure and FCCI resistance of electroplated chromium on HT9 Steel Cladding. Surf. Coat. Technol. 2020, 389, 125652.
- Pina, J.; Dias, A.; François, M.; Lebrun, J.L. Residual stresses and crystallographic texture in hard-chromium electroplated coatings. Surf. Coat. Technol. 1997, 96, 148–162.
- El-Sherik, A.M.; Erb, U. Synthesis of bulk nanocrystalline nickel by pulsed electrodeposition. J. Mater. Sci. 1995, 30, 5743–5749.
- Meng, G.; Sun, F.; Shaoa, Y.; Zhang, T.; Wang, F.; Dong, C.; Li, X. Effect of phytic acid on the microstructure and corrosion resistance of Ni Coating. Electrochim. Acta 2010, 55, 5990–5995.
- Xue, Y.J.; Jia, X.Z.; Zhou, Y.W.; Ma, W.; Li, J.S. Tribological performance of Ni-CeO2 composite coatings by electrodeposition. Surf. Coat. Technol. 2006, 200, 5677–5681.
- Aruna, S.T.; Bindu, C.N.; Ezhil Selvi, V.; William Grips, V.K.; Rajam, K.S. Synthesis and properties of electrodeposited Ni/Ceria nanocomposite coatings. Surf. Coat. Technol. 2006, 200, 6871–6880.
- Han, B.; Lu, X. Tribological and Anti-Corrosion Properties of Ni-W-CeO2 coatings against molten glass. Surf. Coat. Technol. 2008, 202, 3251–3256.
- López, J.R.; Stremsdoerfer, G.; Trejo, G.; Ortega, R.; Pérez, J.J.; Meas, Y. Corrosion Resistance of Nickel Coatings Obtained by Electrodeposition in a Sulfamate Bath in the Presence of Samarium (III). Int. J. Electrochem. Sci. 2012, 7, 12244–12253.
- Makarov, I.; Chen, J.; Yurasovskaya, E.; McCracken, J.; Henein, H. Effect of Electrolyte Additives on Microstructure and Properties of Electroplated Chromium Coatings. Can. Metall. Q. 2011, 50, 153–165.
- Danilov, F.I.; Protsenko, V.S.; Butyrina, T.E.; Vasil’eva, E.A.; Baskevich, A.S. Electroplating of chromium coatings from Cr(III)-Based electrolytes containing water soluble polymer. Prot. Met. 2006, 42, 560–569.
- Protsenko, V.S.; Gordiienko, V.O.; Danilov, F.I.; Kwon, S.C.; Kim, M.; Lee, J.Y. Unusually high current efficiency of nanocrystalline Cr electrodeposition process from trivalent chromium bath. Surf. Eng. 2011, 27, 690–692.
- Mehdipour, N.; Rezaei, M.; Mahidashti, Z. Influence of glycine additive on corrosion and wear performance of electroplated trivalent chromium coating. Int. J. Min. Met. Mater. 2020, 27, 544–554.
- Phuong, N.V.; Kwon, S.C.; Lee, J.Y.; Lee, J.H.; Lee, K.H. The effects of pH and polyethylene glycol on the Cr(III) solution chemistry and electrodeposition of chromium. Surf. Coat. Technol. 2012, 206, 4349–4355.
- Martinuzzi, S.M.; Donati, L.; Giurlani, W.; Pizzetti, F.; Galvanetto, E.; Calisi, N.; Innocenti, M.; Caporali, S. A comparative research on corrosion behavior of electroplated and magnetron sputtered chromium coatings. Coatings 2022, 12, 257.
- Baral, A.; Engelken, R.D. Chromium-based regulations and greening in metal finishing industries in the USA. Environ. Sci. Policy 2002, 5, 121–133.
- Legg, K.O.; Graham, M.; Chang, P.; Rastagar, F.; Gonzales, A.; Sartwell, B. The replacement of electroplating. Surf. Coat. Technol. 1996, 81, 99–105.
- Del Pianta, D.; Frayret, J.; Gleyzes, C.; Cugnet, C.; Dupin, J.C.; Le Hecho, I. Determination of the chromium (III) reduction mechanism during chromium electroplating. Electrochim. Acta 2018, 284, 234–241.
- Srimathi, S.N.; Mayanna, S.M. Electroplating of thin films of magnetic Fe Ni alloys. Mater. Chem. Phys. 1984, 11, 351–364.
- Philippe, L.; Heiss, C.; Michler, J. Electroplating of Stainless Steel. Chem. Mater. 2008, 20, 3377–3384.
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765.
- Barns, S.C.J.; Senderoff, S. Electrodeposition of Alloys. J. Electrochem. Soc. 1964, 111, 296.
- Surviliene, S.; Cesuniene, A.; Selskis, A.; Juskenas, R. Electrodeposition of chromium–cobalt alloy from Cr(III) formate-urea electrolyte. Trans. Inst. Met. Fin. 2010, 88, 100–106.
- Landolt, D.; Marlot, A. Microstructure and composition of pulse-plated metals and alloys. Surf. Coat. Technol. 2003, 169, 8–13.
- Hallez, L.; De Petris-Wery, M.; Assoul, M.; Feki, M.; Ayedi, H. Multicriteria optimization of mechanical and morphological properties of chromium electrodeposits under reverse pulse plating. J. Appl. Electrochem. 2007, 37, 843–852.
- Leisner, P.; Belov, I. Influence of process parameters on crack formation in direct current and pulse reversal plated hard chromium. Trans. Inst. Metal Finish. 2009, 87, 90–96.
- Jin-ku, Y.; Ming-zhi, W.; Qun, L.; Jun, Y.; Lian, L. Effects of saccharin on microstructure and property of electro-deposited Ni-Fe alloys. Trans. Nonferrous Mat. Soc. China 2009, 19, 805–809.
- Clauberg, W.; Schulze-Berge, K. Electrodeposited Chromium—An Overview of Mechanical Properties. Metalloberfläche 1991, 45, 501–505.
- Kobayashi, Y.; Kobayashi, N. Growth-Rate Self-Limitation Mechanism in InP Atomic Layer Epitaxy Studied by Surface Photo-Absorption. J. Surf. Finish. Soc. Jpn. 2005, 56, 334–341.
- Djouani, R.; Qian, X. Mechanism of electrodeposition of nickel. Int. J. Current Res. 2018, 10, 64228–64239.
- Cziraki, A.; Fogarassy, B.F.; Gerocs, I.; Toth-Kadar, E.; Bakonyi, I. Microstructure and growth of electrodeposited nanocrystalline nickel foils. J. Mater. Sci. 1994, 29, 4771–4777.
- Ebrahimi, F.; Morgan, K.L.; Ahmed, Z. The effect of deposition parameters on tensile properties of pulse-plated nanocrystalline nickel. MRS Online Proc. Lib. 2001, 634, 3111.
- Riedel, R.; Bill, J.; Passing, G. A novel carbon material derived from pyridine-borane. Adv. Mater. 1991, 3, 551–552.
- Hagiwara, Y.; Hira, M.; Nishiyama, K.; Ueda, T.; Sakaki, Y.; Ito, T. Developmentally-regulated expression of mNapor encoding an apoptosis-induced ELAV-type RNA binding protein. Proc. Natl. Acad. Sci. USA 1997, 94, 9249–9254. [PubMed]
- Tasuomi, M.; Yasonori, M.; Takahiro, F.; Masaki, I.; Koji, T. Photoreceptor pigment in Blepharisma: H+ release from red pigment. Photo Chem. Photobiol. J. 1992, 3, 399–402.
- Yu, Y.; Sun, L.; Ge, H.; Wei, G.; Jiang, L. Study on electrochemistry and nucleation process of nickel electrodeposition. Int. J. Electrochem. Sci. 2017, 12, 485–495.
- Chen, W.X.; Tu, J.P.; Xu, Z.D.; Chen, W.L.; Zhang, X.B.; Cheng, D.H. Tribological properties of Ni–P-multi-walled carbon nanotubes electroless composite coating. Mater. Lett. 2003, 57, 1256–1260.
- Nitin, P.; Wasekar, A.; Haridoss, P.; Seshadri, S.K.; Sundararajan, G. Influence of mode of electrodeposition, current density and saccharin on the microstructure and hardness of electrodeposited nanocrystalline nickel coatings. Surf. Coat. Technol. 2016, 291, 130–140.
- Puippe, J.C.I.; Ibi, N. Influence of charge and discharge of electric double layer in pulse plating. J. Appl. Electrochem. 1980, 10, 775–784.
- Teschke, O.; Soares, D.M. Electrodeposition of nickel by an asymmetric periodically reversed step current. J. Electrochem. Soc. 1983, 130, 306–310.
- Weil, R. The origins of stress in electrodeposits. Plating 1971, 58, 137.
- Nielsen, C.B.; Leisner, P.; Horsewell, A. On texture formation of chromium electrodeposits. J. Appl. Electrochem. 1998, 28, 141–150.
- Yeo, S.; Kim, J.-H.; Eom, S.-H. The characterization of electrodeposited chromium barriers for nuclear reactor cladding application. J. Nucl. Mater. 2020, 530, 151980.
- Nesnidal, V.L. Qualitative Approach to Pulse Plating. In Proceedings of the International Pulse Plating Symposium, Boston, MA, USA, 19–20 April 1979; AESF Society: Orlando, FL, USA, 1979.
- Knödler, A. Approach to Pulse Plating. In Proceedings of the International Pulse Plating Symposium, Boston, MA, USA, 19–20 April 1979; AESF Society: Orlando, FL, USA, 1979.
- Chin, D.-T.; Zhang, H. A study of pulse plating on chromium. Electrochim. Acta 1986, 3, 299–306.
- Audubert, R. Procédeé d’éLectrolyse. French Patent 932039, 5 May 1948.
- Reby, J.; Vasseur, G.; Sutter, B. Chromium plating in pulse current. In Proceedings of the Conf. Interfinish ’88, Paris, France, 4–7 October 1988; AITE: Stamford, CT, USA, 1988; pp. 369–379.
- Colombini, C. Method for electrolytic deposition of metal layers. Galvanotechnik 1988, 79, 2869–2871.
- Wilson, B.A.; Turley, D.M. Development and characteristics of crack-free chromium coatings produced by electroplating. Trans. IMF 1989, 67, 104–108.
- Badawy, W.A.; Ismail, K.M.; Fathi, A.M. Environmentally safe corrosion inhibition of the Cu–Ni alloys in acidic sulfate solutions. J. Appl. Electrochem. 2005, 35, 879–884.
- Ismail, K.M.; Fathi, A.M.; Badawy, W.A. The influence of Ni content on the stability of copper—Nickel alloys in alkaline sulphate solutions. Appl. Electrochem. 2004, 34, 823–832.
- Li, C.; Li, X.; Wang, Z.; Guo, H. One-pot synthesis of porous nickel cobalt sulphides: Tuning the composition for superior pseudo-capacitance. Rare Metal Mat. Eng. 2015, 44, 1561–1569.
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–506.
- Etaat, M.; Pouraliakbar, H.; Khalaj, G.; Ghambari, M. Adhesion strength measurement of nickel layer on the iron-based P/M parts influenced by different surface pre-treatment operations. Measurement 2015, 66, 204–211.
- Boubatra, M.; Azizi, A.; Schmerber, G.; Dinia, A. The influence of pH electrolyte on the electrochemical deposition and properties of nickel thin films. Ionics 2012, 18, 425–432.
- Goóral, A.; Litynska-Dobrzynska, L.; Kot, M. Effect of surface roughness and structure features on tribological properties of electrodeposited nanocrystalline Ni and Ni/Al2O3 coatings. J. Mater. Eng. Perform. 2017, 26, 2118–2128.
- Swanson, C.J. Roughness in bright nickel electrodeposits. Int. J. Surf. Eng. Coat. 2017, 29, 106–121.
- Péter, L.; Feketea, É.; Kapoor, G.; Gubicza, J. Influence of the preparation conditions on the microstructure of electrodeposited nanocrystalline Ni–Mo alloys. Electrochim. Acta 2021, 382, 138352.
- Mockute, D.; Bernotiene, G. The interaction of additives with the cathode in a mixture of saccharin, 2- butyne-1, 4-diol and phthalimide during nickel electrodeposition in a Watts-type electrolyte. Surf. Coat. Technol. 2000, 135, 42–51.
- Oliveira, E.M.; Finazzi, G.A.; Carlos, I.A. Influence of glycerol, mannitol and sorbitol on electrodeposition of nickel from a Watts bath and on the nickel film morphology. Surf. Coat. Technol. 2006, 200, 597–607.
- Mohanty, U.S.; Tripathy, B.C.; Singh, P.; Das, S.C. Effect of Cd2 on the electrodeposition of nickel from sulfate solutions. Part I: Current efficiency, surface morphology and crystal orientations. J. Electroanal. Chem. 2002, 526, 63–68.
- Oloruntoba, D.; Eghwubare, O.; Oluwole, O. Effect of some process variables on nickel electroplating of low carbon steel, Leonardo El. J. Pract. Technol. 2011, 18, 79–84.
- Agboola, S.O.; Sadiku, E.R.; Biotidara, O.F. The properties and the effect of operating parameters on nickel plating. Int. J. Phys. Sci. 2012, 7, 349.
- Rashidi, A.M.; Amadeh, A. Effect of electroplating parameters on microstructure of nanocrystalline nickel coatings. J. Mater. Sci. Technol. 2010, 26, 82–91.
- Riastuti, R.; Rifki, A.; Herdino, F.; Ramadini, C.; Taruli-Siallagan, S. Effect of saccharin as additive in nickel electroplating on SPCC Steel. AIP Conf. Proc. 2018, 2043, 20012.
- Budi, S.; Manaf, A. The effects of saccharin on the electrodeposition of NiCoFe films on a flexible substrate. Mater. Res. Express 2021, 8, 86513.
- Nakamura, Y. Effects of saccharin and aliphatic alcohols on the electro-crystallization of nickel. J. Appl. Electrochem. 1994, 24, 227–232.
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S. Bright metal coatings from sustainable electrolytes: The effect of molecular additives on electrodeposition of nickel from a deep eutectic solvent. Phys. Chem. Chem. Phys. 2017, 19, 3219–3231.
- Sherwin, C.; Bhat, S. Porosity study on microstructures of nickel-chromium alloy plating on copper substrate. J. Appl. Surf. Interf. 2021, 9, 17–22.
- Zarchi, M.K.; Tahmasebi, F.; Hadipour, A. Enhancment of wear properties of chromium layer by using nickel middle layer. Surf. Eng. Appl. Electrochem. 2021, 57, 448–454.
- Etminanfar, M.R.; Sohi, M.H. Corrosion resistance of multilayer coatings of nanolayered Cr/Ni electrodeposited from Cr(III)–Ni(II) bath. Thin Solid Films 2012, 520, 5322–5327.
- Hsu, L.-S.; Huang, P.-C.; Chou, C.-C.; Hou, K.-H.; Ger, M.-D.; Wang, G.-L. Effect of nickel-phosphorus and nickel-molybdenum coatings on electrical ablation of small electromagnetic rails. Coatings 2020, 10, 1082.
- Sheu, H.-H.; Lu, C.-E.; Lee, H.-B.; Pu, N.-W.; Wu, P.-F.; Hsieh, S.-H.; Ger, M.-D. Electrodeposition of black chromium-cobalt alloy based on trivalent sulfate electrolyte. J. Taiwan Inst. Chem. Eng. 2015, 59, 496–505.
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



































