The introduction of the pulse electroplating method at the beginning of the 21st century and its promising applicability to produce coatings with modified properties have led to its popularity in both scientific and industrial communities.
Original title: Influence of pulse-electroplating parameters on the morphology, structure, chemical composition and corrosion behavior of Co–W alloy coatings.
The relationships between the corrosion behavior of pulse-electroplated cobalt-tungsten coatings and their respective chemical composition, morphological and structural properties (that are affected by pulse-electroplating parameters like peak current density and frequency) are investigated in the present study using scanning electron microscopy (SEM), energy dispersive x-ray analysis (EDX), x-ray diffraction spectroscopy (XRD), potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques, respectively.
The results showed both the coatings' chemical composition and crystal structure can affect their corrosion behaviors; by decreasing pulse frequency (from 5000 to 100Hz) at peak current density of 50 mA/cm2, coatings with higher tungsten content (up to 48.6 wt%), fewer microcracks, coarser crystallite size (up to 57.6 nm) and less lattice microstrain (up to 5.8*10−3) will be deposited which has more corrosion resistance (with corrosion current density of 2.5 μA/cm2). Formation of less defective coatings (with finer crystallite size, up to 52.3 nm, and more lattice microstrain, up to 6.8 × 10−3) including slightly higher tungsten content (up to 49.8 wt%) that have more corrosion resistance occurred at lower applied current densities (25 mA/cm2). So, in the absence of microcracks in the coating's morphology, its tungsten content would be the most effective factor, among other microstructural features, on the coating's corrosion behavior.
1. Introduction
Practical studies on the electrodeposition of tungsten-containing alloy coatings have been carried out extensively due to their good mechanical, tribological, magnetic and electrical properties (Tsyntsaru et al., 2012a). Such properties make them attractive for use in industrial applications where aggressive conditions exist e.g. in aerospace drives and splines where a lubricant may be difficult to maintain (Weston et al., 2010) or a barrier layer for ultra-large-scale integration devices (ULSI) (Tsyntsaru et al., 2012a). Other uses such as application in the fabrication of micro-electromechanical systems (MEMS) (Ehrfeld et al., 1999; Slavcheva et al., 2005) and catalysts for cleaning hydrogen generation from water splitting (Navarro-Flores et al., 2005) have been also attributed to Co–W coatings. Among such tungsten-containing coatings, more attention has been paid to the electrodeposition of Co–W coating due to its superior mechanical properties like high hardness and high wear resistance (comparable with hard chromium coatings as reported previously (Weston et al., 2010); Cr electroplating is conventionally carried out in Cr(VI) baths which are toxic and environmentally unacceptable (Safavi and Walsh, 2021)). The alternatives to hard chromium coatings electrodeposited from Cr (IV) bath might be chromium coatings obtained from Cr (III) solutions, nano-crystalline nickel coatings (Najafi Sayar and Bahrololoom, 2009), alloys and composites, cobalt and coatings formed by plasma electrolytic oxidation method (Brooman, 2004). Practical alternatives to hard chromium that are commonly used in industrial applications are weld overlay of Co alloy (Persson et al., 2003) and Cr3C2–NiCr coatings formed by either thermal spraying (Scrivani et al., 2001) or diffusion processes (Martini et al., 2004); that would be worthy to note that most of them do not fully replace hard chromium, mainly for economic reasons (Vernhes et al., 2013).
Different techniques can be used to synthesize tungsten-containing alloy coatings such as plasma chemical synthesis (V Blagoveshchenskiy et al., 2015), electrical explosion of wire (V Pervikov et al., 2022) and electroplating techniques. The latter would be considered the most versatile technique that would be implemented at atmospheric conditions with relatively low capital costs. DC-electrodeposition of tungsten-containing alloy coatings has been carried out previously where the effects of processing parameters like bath chemistry and temperature have been discussed thoroughly. For instance, the effects of sodium tungstate/cobalt sulfate concentrations in bath and temperature on the resulting Co–W coatings’ properties were investigated to report the optimized processing parameters for electrodeposition of hard Co–W coatings (Abdel Hamid, 2003). In another study, the effect of applied current density on the microhardness values of electrodeposited Co–W coating has been investigated and the results showed that the coatings which were electrodeposited at current densities in the range of 5–10 A/dm2 have microhardness values in the range of 250–550 HV (Oskay, 2022).
Compared to DC-electrodeposition technique, pulse-electrodeposition method has some advantages in the feasibility of electrodeposition of coatings with optimized composition, structure and morphology by controlling applied pulse parameters (like peak current density and frequency), as has been reported previously (Chandrasekar and Pushpavanam, 2008; Melciu and Maidee, 2015). For example, the chemistry and structure of nickel–tungsten coatings (which have been synthesized by pulse electrodeposition technique) have been discussed before (Argañaraz et al., 2012). Moreover, our research group has published a paper on the investigation of the effects of pulse-electrodeposition parameters on the properties of Al coatings that were electrodeposited from molten salt baths; the reported results showed that pulse-electrodeposition parameters affect the resulting coatings’ corrosion and wear properties by influencing their respective morphological and structural characteristics (Arabnejad and Najafisayar, 2022); the same research methodology was applied here to assess the potential influence of pulse-electrodeposition technique on the Co–W coatings that were electrodeposited from aqueous baths.
So far, a number of studies have been carried out to investigate various properties of Co–W coatings such as their structure and thermal stability (Juškėnas et al., 2009), mechanical and tribological properties (Wang et al., 2006), and magnetic properties (Wei et al., 2007). In the case of pulse-electrodeposition of Co–W coatings, a comparison has been made between the tribological properties of coatings that were synthesized by pulse-electrodeposition technique with those that electrodeposited via DC electrodeposition method, the reported results showed that the former ones are more resistive to wear than the latter ones (Su et al., 2013). Moreover, the effect of post-annealing heat treatment on the microstructural features attributed to pulse-electrodeposited Co–W coatings has been discussed elsewhere (Tsyntsaru et al., 2012b). In another study, the effect of the duty cycle on the chemical composition and wear resistance of pulse-electroplated Co–W coatings has been investigated and the results showed that by increasing the coatings’ tungsten content their respective wear resistance will improve (Lu et al., 2018). In another study, the corrosion resistance of electrodeposited Co–W coating was investigated and compared with that of hard chromium; the rereported results showed that corrosion resistance of the former (with specific chemical composition and suitable post-heat treatment) is higher than that of the latter (Capel et al., 2003).
Even though pulse-electrodeposition of Co–W coatings has been investigated thoroughly in the current literature, the relationship between the corrosion behavior of pulse-electroplated coating and its chemical composition, morphology and micro-structure (that are affected by pulse-electroplating parameters) is not appropriately discussed so far. As a result, the present work aims to study the effects of peak current density and pulse frequency on the chemical composition, morphological and structural properties of pulse-electroplated coating and relate them to its corrosion behavior.
2. Material and Methods
2.1. Pulse-electrodeposition of Co–W coatings
Copper plate was used as the substrate with an exposed surface area of 6 cm2. Before each electroplating experiment, the substrate's surface was first mechanically polished with SiC abrasive (grit size of 2000) and then electropolished according to specified conditions that are presented in Table 1. Afterward, the electropolished substrate was washed with distilled water and introduced immediately into a two-electrode cell (as the cathode) containing an electrodeposition bath with the composition that is shown in Table 2. Graphite (with a surface area of 9 cm2) was used as the anode (at distance of 2 cm from cathode) in all electrodeposition experiments and the pH value of the bath was adjusted at 5 using H2SO4 (10 vol%) solution during electrodeposition processes.
Table 1. Parameters of electropolishing process.
| Anode | Cathode | Bath Chemical Comp. | Volume (ml) | Time (min) | Voltage (V) |
| Copper | Stainless steel | Distilled water | 500 | 1–5 | 30–50 |
| Ethanol 95% | 250 | ||||
| Phosphoric acid 85% | 250 |
Table 2. Chemical composition of the electroplating bath.
| Bath constituent | Concentration (g/l) | Role |
| Na2WO4.2H2O | 51 | W ion source |
| CoSO4.7H2O | 40 | Co ion source |
| NH4Cl | 26 | Additive for efficiency enhancement |
| NaBr | 14 | Additive for efficiency enhancement |
| Na3C6H5. 2H2O | 178 | Complexing agent |
| Distilled water | – | Solvent |
All the electrodeposition experiments carried out at room temperature with application of a rectangular pulse wave (only including current on time (Ton) and current off time (Toff) in each period) at duty cycle of 20%; the effects of pulse-electrodeposition parameters, including peak current density (IP: 25 and 50 mA/cm2) and frequency (f: 100, 1000 and 5000 Hz), on the resulting coatings’ properties were investigated. The electrolyte was stirred during electrodeposition experiments using a magnetic stirrer rotating at 260 rpm.
The cathodic current efficiencies of the electrodeposition experiments were determined based on equation (1) (Subramania et al., 2007),
Cathodic current efficiency (%) = M * 100 / ealloy * Q (1)
where M is the mass of the alloy coating (obtained from the difference between initial and coated substrate mass values measured by a precise balance Kern ALS 250-4A), ealloy is the electrochemical equivalent of the alloy and Q is the quantity of electrical charge passed through the electrodeposition cell (54 C). The electrochemical equivalent of the alloy is calculated according to equation (2) (Subramania et al., 2007),
ealloy = eco * ew / (eco • fw) + (ew * fco) (2)
where eCo and eW are the electrochemical equivalents of the constituent metals, 1.1 g/A.h and 1.14 g/A.h for Co and W, respectively, and fCo and fW are their respective weight percent values in the coatings that were obtained from experimental EDX analysis results.
2.2. Coating's morphological and structural characterization
The morphologies and chemical composition of the samples were investigated using a TESCAN-Vega 3 scanning electron microscopy (SEM), Czech Republic, equipped with an energy-dispersive X-ray (EDX) analyzer module. The microstructural analysis of the electrodeposited coatings was carried out by X-ray diffraction (XRD) method; the XRD patterns were obtained in a D8-ADVANCE Bruker spectrometer, Germany, with Cu. kα radiation (1.5 A) and scanning angle (2θ) in the range of 20–90 at a scan rate of 5°/min. Full-width at Half Maximum (Wf) and peak position (θ) attributed to different diffraction peaks were calculated using HighScore Plus software. Williamson-Hall method (Equation (3) (Cullity and Stock, 2001)) was used to fit such calculated values leading to estimated average crystallite size (D) and lattice microstrain (e) values attributed to each respective coating,
Wf = 2e tan θ + [(0.9 * λ) / (D cos θ)] (3)
where λ is the X-ray wavelength.
A microhardness tester (Leitz L137, United Kingdom) was used to measure coatings’ microhardness values by performing indentations at a loading force of 100 g and dwell time of 15 s; five microhardness measurements were conducted and average microhardness value for each sample was reported.
2.3. Coating's corrosion behavior characterization
The corrosion behaviors of the coatings were studied using a potentiostat/galvanostat (Metrohm Autolab III) instrument, Switzerland. A three-electrode electrochemical cell (containing 3.5% NaCl solution at room temperature) was used including the coated substrate as the working electrode, platinum rod as the counter electrode and Ag/AgCl reference electrode. The potentiodynamic polarization tests were carried out at potential rage from −0.3 to +0.3 V with respect to the open circuit potential (OCP) and a potential scan rate of 1 mV/s. Tafel extrapolation technique was used to estimate the corresponding corrosion current density (icorr) values. Electrochemical impedance spectroscopy (EIS) measurements were implemented at OCP using an AC voltage with amplitude of 5 mV and at the frequency range of 100 kHz - 10 mHz, all the electrochemical test results were analyzed by IviumSoft software provided by the potentiostat/galvanostat instrument.
3. Results and Discussion
3.1. Morphological properties of Co–W coatings
SEM micrographs from the surfaces of the coatings that were electrodeposited at various experimental conditions are presented in Fig. 1. The presence of defects such as microcracks in the morphologies of some coatings are significant, especially those that were electrodeposited at high peak current density. The formation of microcracks would be related to high residual stress values in Co–W electrodeposits. The presence of such residual stresses has been related to hydrogen evolution during the electrodeposition process or due to the presence of tungsten in coatings’ chemical composition (Ghaferi et al., 2011; Costa et al., 2020), the same results have been observed by others (Costa et al., 2020; Fathollahzade and Raeissi, 2014). The existence of residual stresses has been also reported in the case of pulse-electrodeposition of other types of alloy coatings. For instance, the estimated residual stress values (based on the Stoney formula) have been reported in the range of 300–1300 MPa in as-deposited Ni–W coatings (Schuh and Ziebell, 2012). By increasing the pulse frequency at constant applied peak current density, microcracks become coarser and the number of pores in the surface increases slightly due to the same reasons, as has been reported elsewhere (Ramaprakash et al., 2021). Last but not least, the coating that was pulse-electroplated at the lowest peak current density and frequency values (25 mA/cm2 and 100 Hz, respectively) includes the least number of defects as can be seen in Fig. 1, that would be related to fewer residual stress values created in coatings which are pulse-electroplated at such experimental conditions as will be discussed below. Typical SEM micrographs from the cross-sections of the coatings that were electrodeposited at various experimental conditions are presented in Fig. 2. As seen, uniform Co–W coatings are electrodeposited on the substrates and an increase in the applied current density results in the formation of thinner films which is in accordance with cathodic current efficiency calculations as will be discussed below. Moreover, no evidence of through-thickness microcracks can be seen in the cross-sectional SEM micrographs indicating that coatings, depending on the electrodeposition conditions, would include some surface microcracks only, as can be seen in Fig. 1. Coatings without any microcracks which reaching to substrate may act as a good barrier against the penetration of corrosive electrolyte to the substrate (Jafari and Sadeghi, 2019).
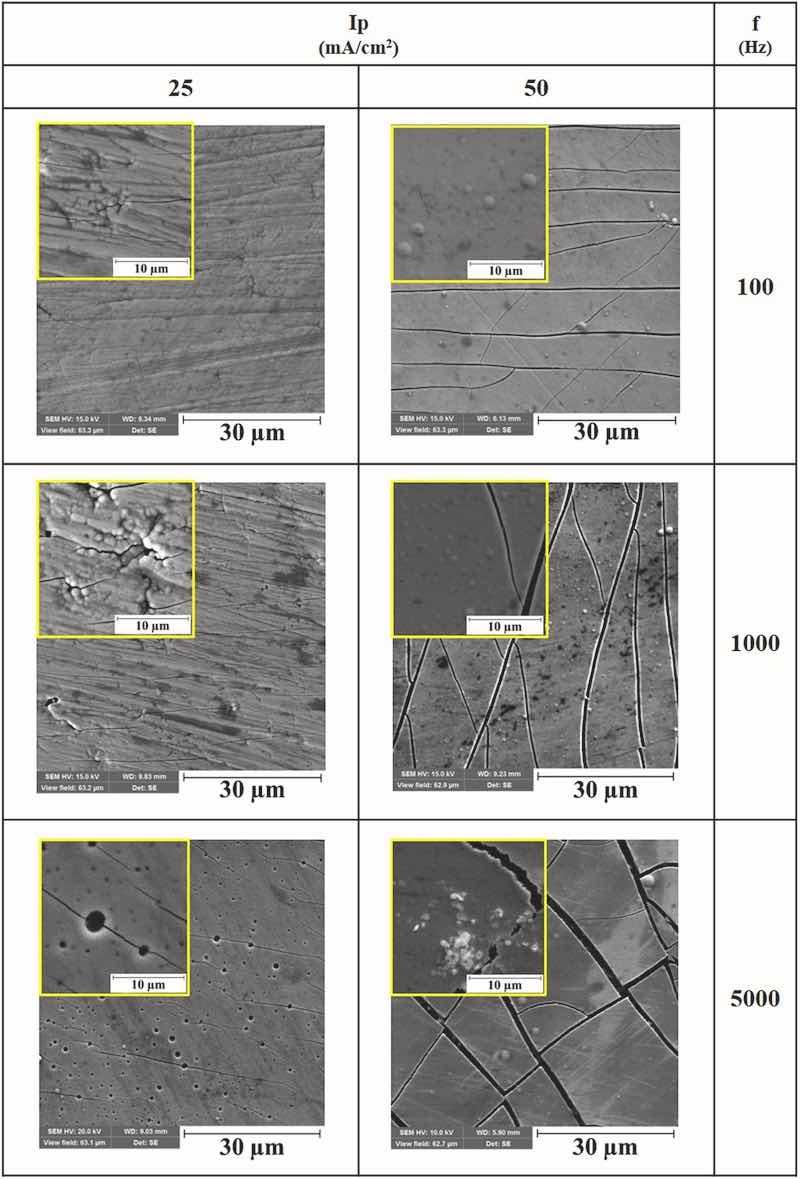
Fig. 1. SEM micrographs from the surfaces of Co–W coatings electrodeposited at various experimental conditions, Ip: Peak current density, f: Pulse frequency.
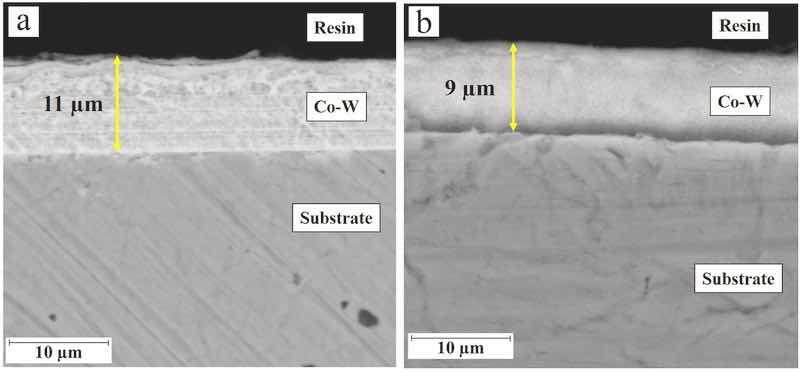
Fig. 2. a) SEM micrographs from the cross-section image of Co–W coating electrodeposited at a) peak current density of 25 mA/cm2 and pulse frequency of 100 Hz, b) peak current density of 50 mA/cm2 and pulse frequency of 100 Hz.
Fig. 3 depicts the influence of pulse electrodeposition parameters (peak current density and pulse frequency values) on the resulting cathodic current efficiency values. As seen, the cathodic current efficiency (CCE) decreases with increasing peak current density and no significant change is noticed in CCE values as the pulse frequency changes during the pulse electrodeposition process. That would be related to enhanced hydrogen evolution at high applied current density values as has been reported previously in the case of potentiostatic electrodeposition of Co–W coatings; the reported results show that by increasing the applied potential during the electrodeposition experiments, cathodic current efficiency decreases and coatings with less W content will be deposited (Ibrahim et al., 2003). It has been reported that current efficiency is related to pulse frequency based on the influence of pulse width on the mechanism of the electrodeposition process. In fact, shortening the pulse period changes the rate-determining step of the electrodeposition phenomenon from charge transfer to diffusion of electroactive species, in this way, since diffusion of hydrogen ions to the cathode surface normally exceeds that of metal cations, so cathodic current efficiency decreases as the pulse frequency increases (Tsai et al., 2002). The decrease in cathodic current efficiency with increasing applied current density has been also reported in the case of Ni–W coatings (Ibrahim et al., 2003; Arunsunai Kumar et al., 2012; Danil’chuk et al., 2018).
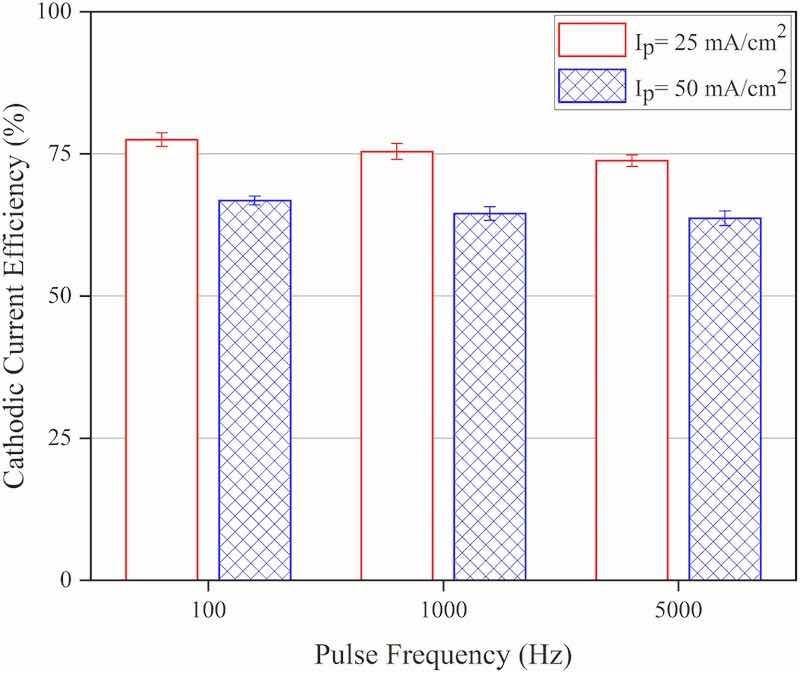
Fig. 3. Cathodic current efficiency values attributed to the coatings that were electrodeposited at various experimental conditions, Ip: Peak current density.
3.2. Structure and chemical composition of Co–W coatings
X-ray diffraction patterns attributed to the coatings that were electrodeposited at various experimental conditions are presented in Fig. 4. As seen, characteristic diffraction peaks at (2θ ≈ 41.3° and 47.1°) which are related to (100), (101) crystalline planes of hcp Co, respectively, (according to JCPDS -05-0727 standard card) are present in the results. Since tungsten is also present in the chemical composition of the coatings (based on EDX analysis results as will be discussed below), that indicates the dissolution of tungsten in cobalt and thus the formation of single-phase solid solution at such experimental conditions. These results are in agreement with those that were reported, previously (Weston et al., 2010; Su et al., 2013) and in accordance with the Co–W phase diagram indicating the stability of ε phase at compositions below 25 at.% and room temperature (Okamoto, 2008).
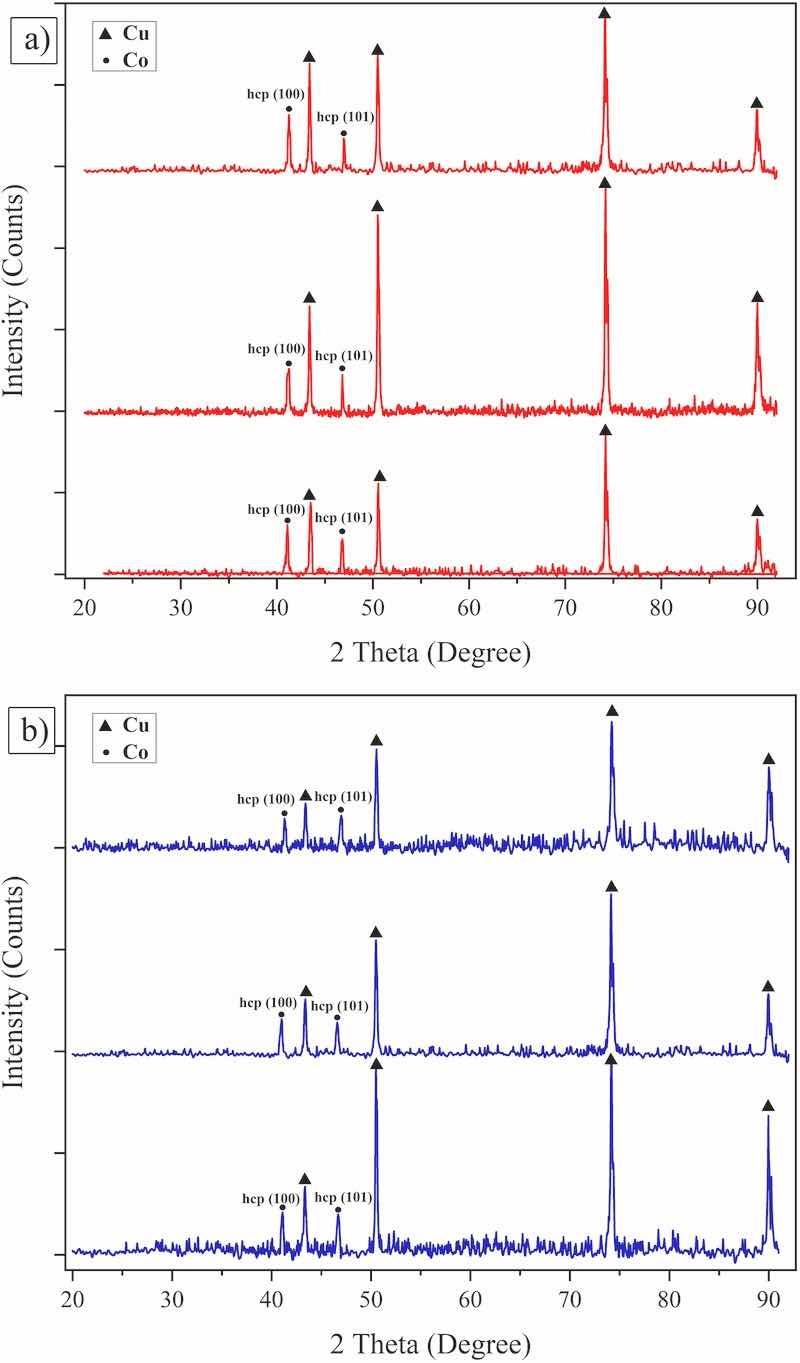
Fig. 4. X-ray diffraction pattern of the coatings that were electrodeposited at peak current density of a) 25 mA/cm2, and b) 50 mA/cm2, f: Pulse frequency.
The estimated values of the crystallite size and lattice microstrain (obtained from such experimental XRD pattern) are presented in Fig. 5. As seen, by increasing pulse frequency (at constant applied peak current density and duty cycle) during electrodeposition process, the average crystallite size of the coating decreases and the amount of residual lattice microstrain increases. In fact, the electrodeposition process consists of nucleation and growth of electrodeposits; at the start of each respective pulse period, the nucleation phenomenon is dominant in comparison with growth leading to promoted nucleation at higher pulse frequencies. Moreover, a thinner diffusion layer would be established near the cathode surface as the pulse frequency increases (having higher ionic concentration at the cathode surface as the pulse frequency increases) promoting the nucleation phenomenon too (Marro et al., 2017; Imanian Ghazanlou et al., 2016). In this regard, coatings with finer crystallite size and more lattice microstrain would be electrodeposited at such experimental conditions. The same behavior has been reported previously in the case of Ni–W pulse-electrodeposition where an increase in pulse frequency has led to the formation of fine crystallites in the final coatings (Detor and Schuh, 2007; Wasekar et al., 2020). Last but not least, a comparison between data presented in Fig. 5 and coatings' surface morphologies (Fig. 1) reveals that those coatings that were electrodeposited at high applied peak current densities and frequencies include more residual stress and microcracks. The same behavior has been also reported in the case of pulse electrodeposition of Ni–W coatings where the increase in applied pulse frequency has led to the formation of coatings with more microcracks (Ramaprakash et al., 2021) and also in galvanostatic electrodeposition of Co–W coatings where more defective coatings have been formed at higher applied current densities (Costa et al., 2020). On the other hand, by increasing peak current density (at constant pulse frequency), the average crystallite size increases and microstrain decreases, in other words, although it would be expected to obtain coatings with finer crystallite size values at higher applied peak current densities, coatings with larger crystallite sizes are formed at such experimental conditions. So, contrary to the above observations, the coatings' tungsten content seems to be more effective on the resulting crystallite size values than the applied peak current density. The dominant effect of coating chemical composition (rather than electrodeposition conditions) on the resulting coatings' crystallite size values has been also reported previously; for instance, an increase in coatings’ tungsten content was reported to be accompanied by the formation of coating with smaller crystallite size in the case of Ni–W (Detor and Schuh, 2007; Wasekar et al., 2020) and Co–W (Tsyntsaru et al., 2012b, 2013) electrodeposition processes.
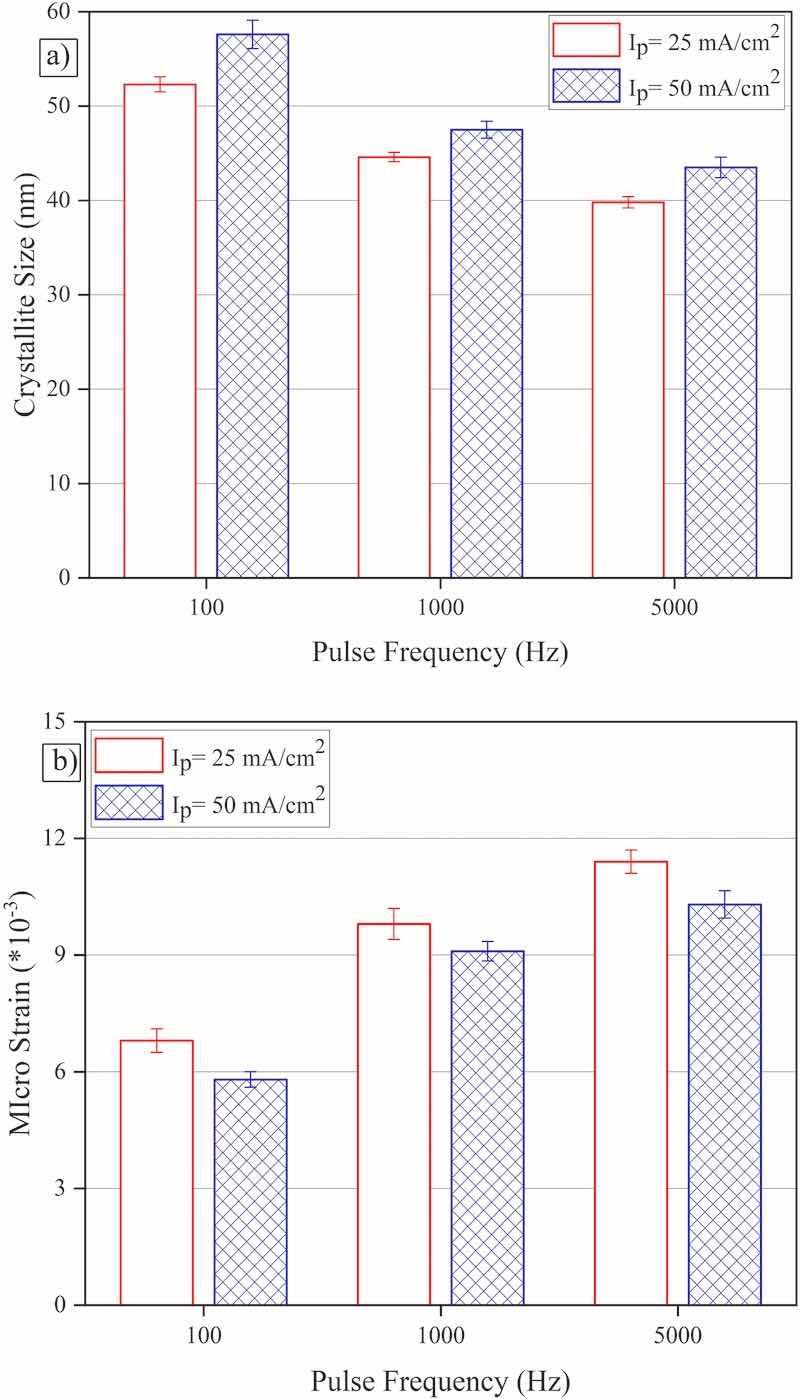
Fig. 5. a) Average crystallite sizes, and b) lattice microstrain values attributed to the coatings that were electrodeposited at various experimental conditions Ip: Peak current density.
Fig. 6 shows the variation of microhardness values attributed to the coatings that were electrodeposited at various experimental conditions. As seen, by increasing the current density (at constant frequency) the hardness of the resulting coating decreases significantly. That would be related to a decrease in tungsten content (as will be discussed below) and an increase in average crystallite size attributed to the coatings that were electrodeposited at such experimental conditions. As has been reported before (Su et al., 2013), with increasing the current density, less tungsten will be co-deposited with cobalt to form the final Co–W coatings having fewer microhardness values. The same trend is observed in the case of increasing applied pulse frequency at constant peak current density values during electrodeposition experiments, i.e., the coating's microhardness decreases slightly as the pulse frequency increases. It would be related to the decrease in tungsten content of the coatings that were electrodeposited at such experimental conditions in spite of their smaller crystallite size values compared to those that were electrodeposited at lower frequencies. Another fact that would be noted from the microhardness results is that those coatings that have fewer lattice microstrain values (specifically electrodeposited at lower pulse frequencies) have higher microhardness values; it would be related to the formation of fewer defective coatings (including fewer surface microcracks) at such pulse-electrodeposition conditions, such fewer defective coatings provide more material to resist local plastic deformation, so exhibiting higher microhardness values (Ma et al., 2006).
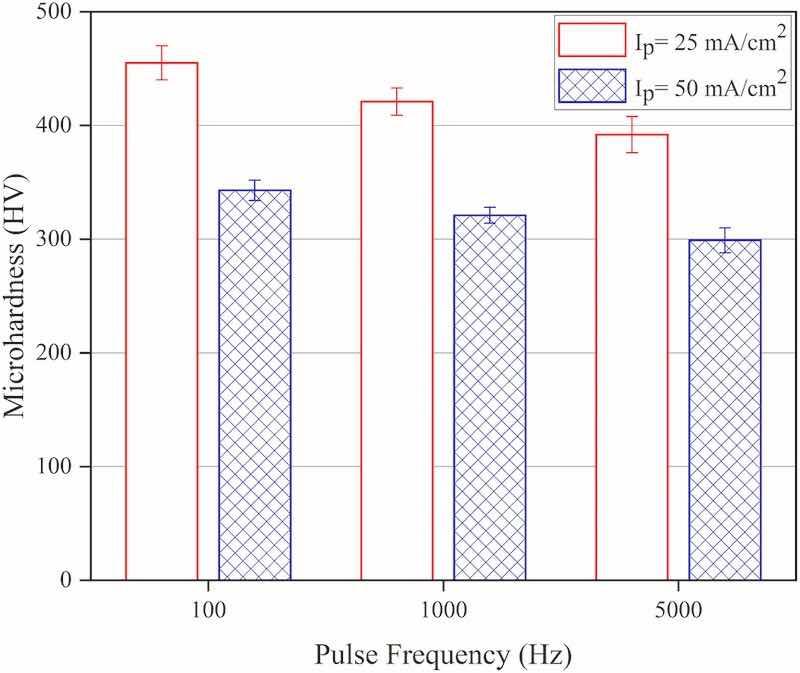
Fig. 6. Microhardness values attributed to the coatings that were electrodeposited at various experimental conditions, Ip: Peak current density.
The microhardness values vary between 300 and 450 HV, attributed to the coatings that were electrodeposited at various experimental conditions, in spite of the fact that these values seem to be out of range of standard microhardness values attributed to Co–W coatings (400–800 HV) but they are actually in agreement with various previously reported microhardness values of Co–W coatings (250–720 HV) that were electrodeposited at different experimental conditions in (Oskay, 2022; Lu et al., 2018; Ghaferi et al., 2011; Brenner and Brenner, 1963); such discrepancies would be related to the effect substrate (in this case, copper) on the microhardness values when measurements implemented on thin hard coatings electroplated on soft substrates.
The prominent effect of higher tungsten content, as the alloying element, to increase the coating's microhardness value (compared to other effective factors such as fine crystallite size) has been also reported previously (Weston et al., 2010; Tsyntsaru et al., 2012b; Bodaghi and Hosseini, 2012). Moreover, the effect of chemical composition on the hardness of ternary W–B–C coating has been reported; the reported results showed that tungsten content is the key parameter influencing coatings' microhardness values, harder coating has been reported to contain more W in its chemical composition (Mirzaei et al., 2020).
Typical EDX analysis results of the Co–W coatings that were pulse electrodeposited at various experimental conditions are presented in Fig. 7. As seen, Co and W elements are distributed uniformly through the thickness of the coatings indicating the formation of homogenous Co–W alloy coatings at such experimental conditions. The corresponding elemental compositions attributed to the coatings is presented in Fig. 8. The results show that an increase in peak current density (at constant frequency) has led to a decrease in tungsten concentration in the chemical composition of coatings. It has been reported that electrodeposition of Co–W coatings occurs via sequential electrochemical/chemical reactions taking place at the cathode surface as shown in equations (4), (5) (Ibrahim et al., 2003).
WO2/4− + 4H2O + 2 e− → WO2. 2H2O + 4OH− (4)
WO2.2H2O + 4H-(Co) → Co–W + 4H2O (5)
where tungstate ions are first electrochemically reduced to tungsten oxide followed by chemical reduction of the latter to W atoms by the aid of catalytic action of reduced Co atoms at the cathode surface (Ibrahim et al., 2003). In spite of the fact that electrochemical reduction of WO2/4− ions would remain as an activation-controlled phenomenon at high applied current densities, but mechanism of electrochemical reduction of Co2+ions would change to diffusion-controlled one at such experimental conditions (high applied current densities) (Ghaferi et al., 2011). In this regard, fewer cobalt atoms (catalyst agents available for the production of W atom entering the growing coating at the cathode surface) would be available at high applied current densities leading to the formation of coatings with less W-content compared to those that electrodeposited at low peak current density values (Fig. 8). Such observations have been also reported in the case of galvanostatic (Ma et al., 2017) and potentiodynamic (Ibrahim et al., 2003) electrodeposition of Co–W coatings and also in the electrodeposition of Ni–W coatings (Moussa et al., 2006; Eliaz et al., 2005). Another fact that can be seen in Fig. 8 is that by increasing the frequency at constant applied peak current density, the concentration of tungsten in coatings will decrease. That would be related to the shortage of available time (Ton) for the occurrence of sequential electrochemical/chemical reactions required for tungsten incorporation to growing coatings at such experimental conditions (Puippe et al., 1986). The same behavior has been also reported in the case of pulse electrodeposition Co–W coatings where fewer tungsten atoms were incorporated into the coatings at high pulse frequency values (Mulukutla et al., 2012).
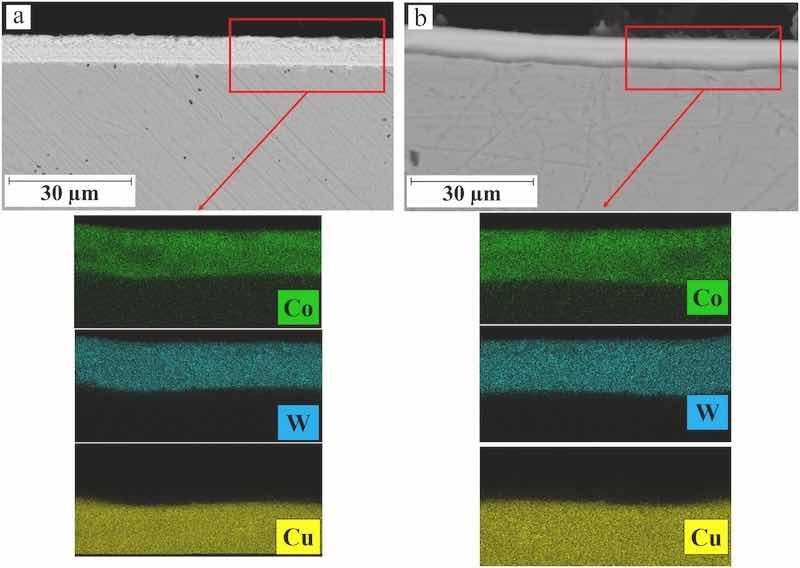
Fig. 7. a) EDX mapping from the cross-section image of Co–W coating electrodeposited at a) peak current density of 25 mA/cm2 and pulse frequency of 100 Hz, b) peak current density of 50 mA/cm2 and pulse frequency of 100 Hz.
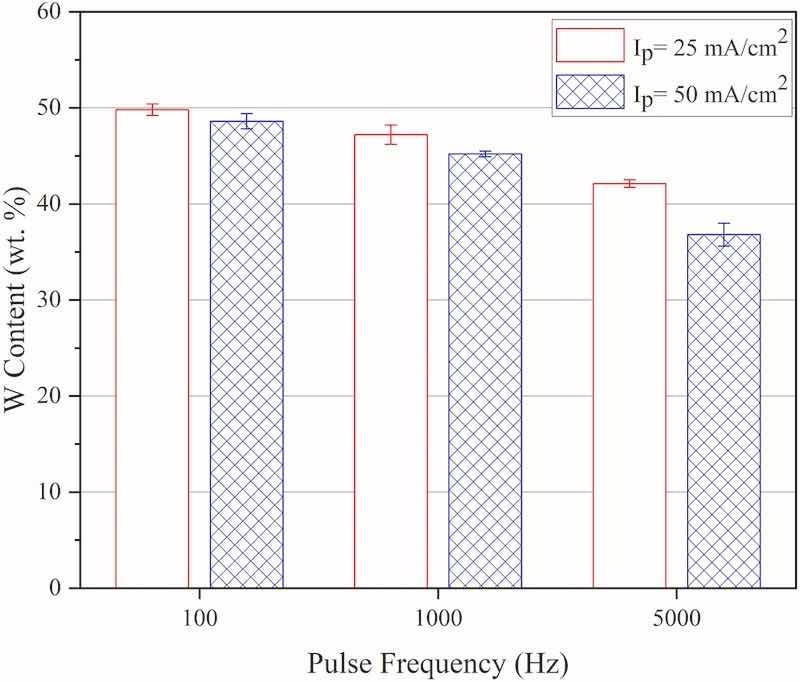
Fig. 8. Tungsten content of the coatings that were electrodeposited at various experimental conditions, Ip: Peak current density.
3.3. Corrosion behavior of Co–W coatings
Typical potentiodynamic polarization curves attributed to the coatings that were electrodeposited at different experimental conditions are presented in Fig. 9. As seen, all the samples exhibited active corrosion behavior at such test conditions; the respective estimated corrosion current density values (Icorr) and corrosion potential (Ecorr) are presented in Table 3. As seen, the corrosion current density (corrosion rates) that is attributed to bare substrate is less than those of Co–W coatings that were electrodeposited at different experimental conditions; that would not be interesting result since the substrate metal (copper) in generally more resistant to corrosion than Co–W alloy coatings that are constituted from active metals like cobalt and tungsten. In the case of Co–W coatings, the corrosion resistance decreases (corrosion rate increases) as the pulse frequency increases (at constant applied peak current density) during the pulse-electrodeposition process. That would be related to a decrease in tungsten content (Fig. 8) and an increase in microcracks (Fig. 1) in the coatings that were electrodeposited at such experimental conditions. In addition, the same trend in the coatings' corrosion resistance can be observed in the case of increasing applied peak current density (at constant pulse frequency) during their pulse-electrodeposition process. That would be related to the same reason that said above, i.e., with increasing peak current density at constant pulse frequency, the coatings with less tungsten content and more microcracks will be electrodeposited. The corrosion potentials (Ecorr) depict the same results; by looking into potentiodynamic polarization curves (Fig. 9) and estimated corrosion potential (Table .3), the corrosion potentials shift to more positive values (more noble) as the pulse frequency decreases (from 5000 to 100 Hz at constant applied peak current density) during the pulse-electrodeposition process. Among all pulse-electroplated Co–W coatings, the lowest corrosion current density (1.9 μA/cm2) and more noble corrosion potential (−664 mV) was observed for the coating deposited using 25 (mA/cm2) peak current density and 100 Hz frequency. Coatings that were electrodeposited at various experimental conditions depict corrosion current densities (Icorr) in the range of 1.9–6.3 μA/cm2, such values are typically lower than previously reported corrosion current densities, i.e. (4.5–15 μA/cm2), attributed to Co–W coatings that were electrodeposited in various experimental conditions (Su et al., 2013; Ghaferi et al., 2011; Fathollahzade and Raeissi, 2014; Bodaghi and Hosseini, 2012). Generally speaking, the coatings’ corrosion resistance is commonly affected by their respective chemical compositions and morphology. For instance, it has been reported that by increasing the tungsten content the corrosion resistance increases in the case of iron group-based alloy coatings (Królikowski et al., 2009); such phenomenon has been related to the formation of more protective films on the surface of coatings with higher tungsten (Su et al., 2013; Sriraman et al., 2007). Moreover, the presence of microcracks would lead to the occurrence of localized corrosion which in turn causes to have high corrosion currents in polarization test results (Jiao et al., 2018). The same results have been reported in the case of electrodeposition of Ni–W coatings (Królikowski et al., 2009) and also in Fe-based amorphous coatings (Jiao et al., 2018) where coatings with more microcracks exhibited higher corrosion rates than the intact ones.
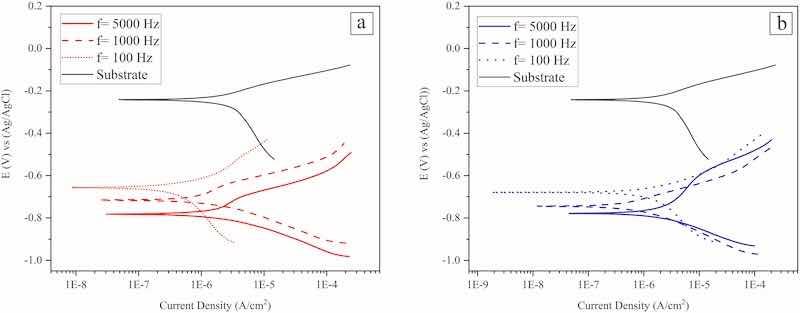
Fig. 9. Potentiodynamic polarization curves attributed to the coatings that were electrodeposited at peak current density of a) 25 mA/cm2, and b) 50 mA/cm2, f: Pulse frequency.
Table 3. Fitted results to experimental electrochemical impedance spectroscopy (EIS) data and potentiodynamic polarization (PDP) results. First 2 columns are "Coating pulse-electroplating parameters," the next four are "EIS," and final two are "PDP."
| Peak current density (mA/cm2) | Frequency (Hz) | Rct (Ω/cm2) | Rs (Ω/cm2) | Q (mF.sn−1/cm2) | n | Ecorr (mV) | Icorr (μA/cm2) |
| 25 | 100 | 3224 | 12.51 | 2.372 | 0.80 | −664 | 1.9 |
| 1000 | 1719 | 11.94 | 4.034 | 0.76 | −723 | 3.8 | |
| 5000 | 1125 | 11.12 | 5.416 | 0.71 | −781 | 5.4 | |
| 50 | 100 | 2210 | 14.02 | 3.274 | 0.77 | −687 | 2.5 |
| 1000 | 1213 | 13.63 | 5.512 | 0.70 | −759 | 4.6 | |
| 5000 | 712 | 12.06 | 7.298 | 0.61 | −793 | 6.3 |
Fig. 10 shows Nyquist plots attributed to the Co–W coatings that were electrodeposited at various experimental conditions. As seen, all the coatings exhibit AC responses including depressed semi-circles that is typical behavior of free corroding bare metal in a corrosive medium. Such an AC response includes a single time constant that is attributed to charge transfer phenomenon taking place at the working electrode interface with the electrolyte under activation-controlled corrosion. In fact, this type of AC response is the result of capacitive behavior of the electrochemical double layer which is parallel with the electrode resistance to charge transfer. This behavior results in having semi-circles with different diameters in EIS Nyquist plots insomuch as different electrodes would have different resistance to charge transfer with electroactive species in the electrolyte (Raistrick et al., 2005; Tait, 1994). The equivalent circuit that was used to fit the experimental EIS data (Fig. 10) is presented in Fig. 11 in which Rs is the solution resistance, Q is related to double layer capacitance, n is phenomenological coefficients and Rct is charge transfer resistance (or corrosion resistance).
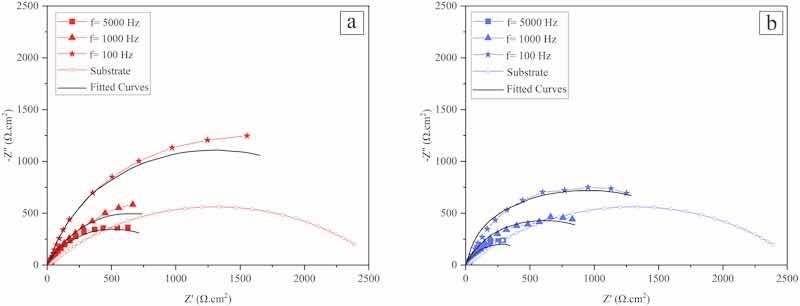
Fig. 10. Nyquist plots of the coatings that were electrodeposited at current density of a) 25 mA/cm2, and b) 50 mA/cm2, f: Pulse frequency.
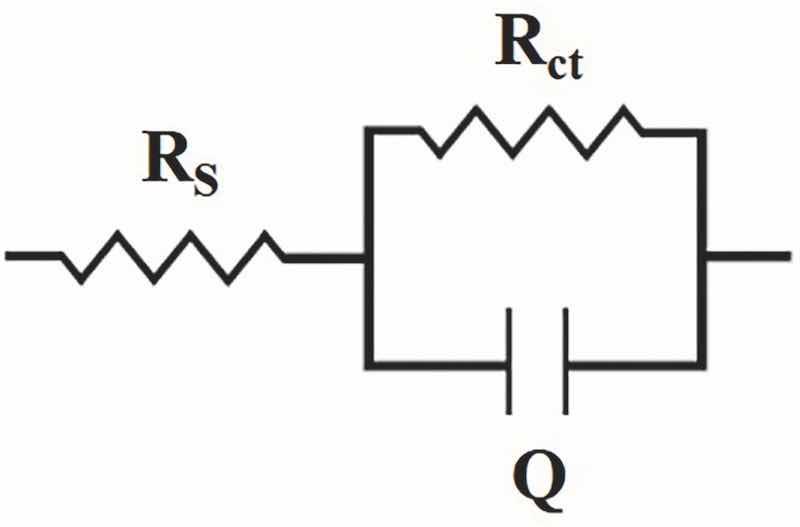
Fig. 11. The equivalent circuit that were used to fit experimental EIS data shown in Fig. 11.
The fitted results to experimental EIS data are given in Table 3, as seen, by increasing pulse frequency (at constant applied peak current density) or increasing the peak current density (at constant applied pulse frequency) the Rct values decrease (their respective corrosion resistance decreases) which is in accordance with polarization test results. It has been reported that as the electrode surface becomes smoother and perfectly flat, the value of the “n” parameter approaches 1 (Tait, 1994). In the case of fitted values to the “n” parameter that is presented in Tables 3 and it can be seen that by decreasing pulse frequency and applying peak current density the value of “n” increases, which would be related to the formation of fewer defective coatings (including fewer surface cracks) at such experimental conditions. Moreover, the value of Q is related to double-layer capacitance which decreases as the charge transfer resistance increases, the latter describes the kinetics of faradaic current passing through the electrode interface and the former is related to electrode double-layer capacitive current (McKubre et al., 2018). In this way, as the charge transfer resistance decreases the value of corrosion current densities increases; it is in accordance with the trend of electrochemical data that are presented in Table 3.
4. Conclusions
The effects of pulse frequency and peak current density on the chemical composition, morphology, crystal structure and the resulting corrosion behavior of Co–W coatings were investigated. The results showed that as the pulse frequency decreases (from 5000 to 100 Hz at constant applied peak current density) coating tungsten content increases (approximately 9 wt %), its crystallite size increases, its lattice microstrain decreases and fewer microcracks will be observed in the morphology of the resulting coatings. Such characteristics will lead to have Co–W coating with more corrosion resistance in comparison to those that were electrodeposited at high pulse frequency values. The same trend was observed in the case of decreasing the applied peak current density (from 50 to 25 mA/cm2 at constant pulse frequency) where more corrosion resistance can be attributed to those coatings that were electrodeposited at lower peak current density values; such coatings were less defective, include more tungsten content but have finer crystallite size and more lattice microstrain compared to those that were electrodeposited at higher peak current densities. In this regard, since both the coating's chemical composition (its W content) and crystal structure (crystallite size and lattice microstrain) can affect its corrosion behavior (i.e., higher W content, coarser crystallite size and less lattice microstrain make the coating more resistive to corrosion) but the former (chemical composition) is more effective than the latter one. To sum up, the Co–W coating which was electrodeposited at a peak current density of 25 mA/cm2 and pulse frequency of 100 Hz includes the highest tungsten content (49.8 wt%) that gave it the highest microhardness value (460 HV) and lowest corrosion rate (iCorr = 1.9 μA/cm2) among the other ones. Co–W alloy coatings; high tungsten content and low surface defect improve Co–W coatings corrosion and mechanical properties that would make them more suitable in various industrial applications.
Authors Mohammad Milad Zehtab and Pooria Najafisayar are with the Department of Materials Science and Engineering, School of Engineering, Shiraz University, Shiraz, Iran
Credit authorship contribution statement: M. Zehtab: Writing – original draft, Formal analysis, Data curation. P. Najafisayar: Supervision, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement: Authors would like to thank Shiraz University for its financial support to do this research work (Grant number: 97GCU1M192010).
Data availability: Data will be made available on request.
References
Abdel Hamid, Z., 2003. Electrodeposition of cobalt–tungsten alloys from acidic bath containing cationic surfactants. Mater. Lett. 57, 2558–2564. https://doi.org/ 10.1016/S0167-577X(02)01311-3.
Arabnejad, A., Najafisayar, P., 2022. Pulse electrodeposition of aluminium coatings from molten salt bath; effect of processing parameters on the coatings’ corrosion and wear behaviour. Trans. IMF. 100, 305–312. https://doi.org/10.1080/ 00202967.2022.2095112.
Arganaraz, M.P.Q., Ribotta, S.B., Folquer, M.E., Zelaya, E., Llorente, C., Ramallo- Lopez, J.M., Benítez, G., Rubert, A., Gassa, L.M., Vela, M.E., Salvarezza, R.C., 2012. The chemistry and structure of nickel–tungsten coatings obtained by pulse galvanostatic electrodeposition. Electrochim. Acta 72, 87–93. https://doi.org/ 10.1016/j.electacta.2012.03.163.
Arunsunai Kumar, K., Paruthimal Kalaignan, G., Muralidharan, V.S., 2012. Pulse electrodeposition and characterization of nano Ni–W alloy deposits. Appl. Surf. Sci. 259, 231–237. https://doi.org/10.1016/j.apsusc.2012.07.024.
Bodaghi, A., Hosseini, J., 2012. Corrosion behavior of electrodeposited cobalt-tungsten alloy coatings in NaCl aqueous solution. Int. J. Electrochem. Sci. 7.
Brenner, A., 1963. 33 - electrodeposition of tungsten alloys containing cobalt. In: Brenner, A.B.T.-E. of A. (Ed.), Nickel, And/or Iron. Academic Press, pp. 347–412. https://doi.org/10.1016/B978-1-4831-9807-1.50024-6.
Brooman, E.W., 2004. Wear behavior of environmentally acceptable alternatives to chromium coatings: cobalt-based and other coatings. Met. Finish. 102, 42–54. https://doi.org/10.1016/S0026-0576(04)84659-2.
Capel, H., Shipway, P.H., Harris, S.J., 2003. Sliding wear behaviour of electrodeposited cobalt–tungsten and cobalt–tungsten–iron alloys. Wear 255, 917–923. https://doi.org/10.1016/S0043-1648(03)00241-2.
Chandrasekar, M.S., Pushpavanam, M., 2008. Pulse and pulse reverse plating—conceptual, advantages and applications. Electrochim. Acta 53, 3313–3322. https://doi.org/10.1016/j.electacta.2007.11.054.
Costa, J.M., Porto, M.B., Amancio, R.J., de Almeida Neto, A.F., 2020. Effects of tungsten and cobalt concentration on microstructure and anticorrosive property of cobalt- tungsten alloys. Surface. Interfac. 20, 100626 https://doi.org/10.1016/j.surfin.2020.100626.
Cullity, B.D., Stock, S.R., 2001. Elements of X-Ray Diffraction, third ed. Prentice Hall, Upper Saddle River, NJ, Upper Saddle River, NJ SE - xviii, p. 664. illustrations ; 25 cm. https://worldcat.org/title/46437243.
Detor, A.J., Schuh, C.A., 2007. Tailoring and patterning the grain size of nanocrystalline alloys. Acta Mater. 55, 371–379. https://doi.org/10.1016/j.actamat.2006.08.032.
Ehrfeld, W., Hessel, V., L¨ owe, H., Schulz, C., Weber, L., 1999. Materials of LIGA technology. Microsyst. Technol. 5, 105–112. https://doi.org/10.1007/ s005420050150.
Eliaz, N., Sridhar, T.M., Gileadi, E., 2005. Synthesis and characterization of nickel tungsten alloys by electrodeposition. Electrochim. Acta 50, 2893–2904. https://doi.org/10.1016/j.electacta.2004.11.038.
Fathollahzade, N., Raeissi, K., 2014. Electrochemical evaluation of corrosion and tribocorrosion behaviour of amorphous and nanocrystalline cobalt–tungsten electrodeposited coatings. Mater. Chem. Phys. 148, 67–76. https://doi.org/10.1016/ j.matchemphys.2014.07.013.
Ghaferi, Z., Raeissi, K., Golozar, M.A., Edris, H., 2011. Characterization of nanocrystalline Co–W coatings on Cu substrate, electrodeposited from a citrate- ammonia bath. Surf. Coatings Technol. 206, 497–505. https://doi.org/10.1016/j.surfcoat.2011.07.074.
Ibrahim, M.A.M., El Rehim, S.S.A., Moussa, S.O., 2003. Electrodeposition of noncrystalline cobalt–tungsten alloys from citrate electrolytes. J. Appl. Electrochem. 33, 627–633. https://doi.org/10.1023/A:1024916903544.
Imanian Ghazanlou, S., Shokuhfar, A.L.I., Navazani, S., Yavari, R., 2016. Influence of pulse electrodeposition parameters on microhardness, grain size and surface morphology of Ni–Co/SiO2 nanocomposite coating. Bull. Mater. Sci. 39, 1185–1195. https://doi.org/10.1007/s12034-016-1256-1.
Jafari, R., Sadeghi, E., 2019. High-temperature corrosion performance of HVAF-sprayed NiCr, NiAl, and NiCrAlY coatings with alkali sulfate/chloride exposed to ambient air. Corros. Sci. 160, 108066 https://doi.org/10.1016/j.corsci.2019.06.021.
Jiao, J., Luo, Q., Wei, X., Qu, S., Wang, Y., Shen, J., 2018. Effect of microcracks on the corrosion behaviour of Fe-based amorphous coatings in chloride solutions. Int. J. Electrochem. Sci. 13, 5522–5534. https://doi.org/10.20964/2018.07.63.
Juskenas, R., Valsiunas, I., Pakstas, V., Giraitis, R., 2009. On the state of W in electrodeposited Ni–W alloys. Electrochim. Acta 54, 2616–2620. https://doi.org/ 10.1016/j.electacta.2008.10.060.
Krolikowski, A., Płonska, E., Ostrowski, A., Donten, M., Stojek, Z., 2009. Effects of compositional and structural features on corrosion behavior of nickel–tungsten alloys. J. Solid State Electrochem. 13, 263–275. https://doi.org/10.1007/s10008- 008-0712-2.
Lu, H., Qin, J., Di, C., Yang, Y., Huo, R., 2018. Effect of duty cycle on microstructure, tungsten content and wear resistance of tungsten-cobalt films prepared by electrodeposition. Mater. Trans. 59, 1943–1948. https://doi.org/10.2320/ matertrans.M2018196.
Ma, C.-H., Huang, J.-H., Chen, H., 2006. Nanohardness of nanocrystalline TiN thin films. Surf. Coatings Technol. - SURF COAT TECH. 200, 3868–3875. https://doi.org/ 10.1016/j.surfcoat.2004.10.098.
Ma, L., Xi, X., Nie, Z., Dong, T., Mao, Y., 2017. Electrodeposition and characterization of Co-W alloy from regenerated tungsten salt. Int. J. Electrochem. Sci. 12, 1034–1051. https://doi.org/10.20964/2017.02.37.
Marro, J.B., Darroudi, T., Okoro, C.A., Obeng, Y.S., Richardson, K.C., 2017. The influence of pulse plating frequency and duty cycle on the microstructure and stress state of electroplated copper films. Thin Solid Films 621, 91–97. https://doi.org/10.1016/j.tsf.2016.11.047.
Martini, C., C, G., Poli, G., Prandstraller, D., 2004. Sliding and abrasive wear behaviour of boride coatings. Wear 256, 608–613. https://doi.org/10.1016/j.wear.2003.10.003.
McKubre, M.C.H., Macdonald, D.D., Sayers, B., Macdonald, J.R., 2018. Measuring techniques and data analysis. In: Impedance Spectrosc, pp. 107–174. https://doi.org/10.1002/9781119381860.ch3.
Melciu, D., Maidee, N., 2015. Pulse-electroplating: Process Parameters and Their Influence on the Formed Microstructure.
Mirzaei, S., Alishahi, M., Souˇ cek, P., ˇ Zeníˇ sek, J., Holec, D., Koutn´ a, N., Burˇ síkov´ a, V., Stupavsk´ a, M., Z´ abranský, L., Burmeister, F., Blug, B., Czig´ any, Z., Bal´ azsi, K., Mikˇ sov´ a, R., Vaˇ sina, P., 2020. The effect of chemical composition on the structure, chemistry and mechanical properties of magnetron sputtered W-B-C coatings: modeling and experiments. Surf. Coatings Technol. 383, 125274 https://doi.org/ 10.1016/j.surfcoat.2019.125274.
Moussa, S.O., Ibrahim, M.A.M., El Rehim, S.S.A., 2006. Induced electrodeposition of tungsten with nickel from acidic citrate electrolyte. J. Appl. Electrochem. 36, 333–338. https://doi.org/10.1007/s10800-005-9069-8.
Mulukutla, M., Kommineni, V.K., Harimkar, S.P., 2012. Pulsed electrodeposition of Co–W amorphous and crystalline coatings. Appl. Surf. Sci. 258, 2886–2893. https:// doi.org/10.1016/j.apsusc.2011.11.002.
Najafi Sayar, P., Bahrololoom, M.E., 2009. Tribological properties of pulse plated nanocrystalline nickel coatings as environmentally accepted alternative to conventional chromium coatings. Trans. IMF. 87, 246–253. https://doi.org/ 10.1179/174591909X439964.
Navarro-Flores, E., Chong, Z., Omanovic, S., 2005. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. Chem. 226, 179–197. https://doi.org/10.1016/j.molcata.2004.10.029.
Okamoto, H., 2008. Co-W (Cobalt-Tungsten). J. Phase Equilibria Diffus. 29, 119. https:// doi.org/10.1007/s11669-007-9229-0.
Oskay, K., 2022. Effects of pH and current density on microstructure and hardness of the cobalt-tungsten coating. Adıyaman Üniversitesi Mühendislik Bilim. Derg 9. https:// doi.org/10.54365/adyumbd.1007722.
Persson, D.H.E., Jacobson, S., Hogmark, S., 2003. Effect of temperature on friction and galling of laser processed Norem 02 and Stellite 21. Wear 255, 498–503. https://doi.org/10.1016/S0043-1648(03)00122-4.
Puippe, J.-C., Leaman, F., American, E., Surface Finishers, S., 1986. Theory and Practice of Pulse Plating. American Electroplaters and Surface Finishers Society, Orlando, Fla., Orlando, Fla.
Raistrick, I.D., Franceschetti, D.R., Macdonald, J.R., 2005. Theory. In: Impedance Spectrosc, pp. 27–128. https://doi.org/10.1002/0471716243.ch2.
Ramaprakash, M., Deepika, Y., Balamurugan, C., Nagaganapathy, N., Sekar, R., Panda, S.K., Rajasekaran, N., 2021. Pulse electrodeposition of nano-crystalline Ni-W alloy and the influence of tungsten composition on structure, microhardness and corrosion properties. J. Alloys Compd. 866, 158987 https://doi.org/10.1016/j.jallcom.2021.158987.
Safavi, M.S., Walsh, F.C., 2021. Electrodeposited Co-P alloy and composite coatings: a review of progress towards replacement of conventional hard chromium deposits. Surf. Coatings Technol. 422, 127564 https://doi.org/10.1016/j.surfcoat.2021.127564.
Schuh, C.A., Ziebell, T.D., 2012. Residual stress in electrodeposited nanocrystalline nickel-tungsten coatings. J. Mater. Res. 27, 1271–1284, 10.1557/jmr.2012.51.
Scrivani, A., Ianelli, S., Rossi, A., Groppetti, R., Casadei, F., Rizzi, G., 2001. A contribution to the surface analysis and characterisation of HVOF coatings for petrochemical application. Wear 250, 107–113. https://doi.org/10.1016/S0043- 1648(01)00621-4.
Slavcheva, E., Mokwa, W., Schnakenberg, U., 2005. Electrodeposition and properties of NiW films for MEMS application. Electrochim. Acta 50, 5573–5580. https://doi.org/ 10.1016/j.electacta.2005.03.059.
Sriraman, K.R., Ganesh Sundara Raman, S., Seshadri, S.K., 2007. Corrosion behaviour of electrodeposited nanocrystalline Ni–W and Ni–Fe–W alloys. Mater. Sci. Eng. A. 460–461, 39–45. https://doi.org/10.1016/j.msea.2007.02.055.
Su, F., Liu, C., Huang, P., 2013. Establishing relationships between electrodeposition techniques, microstructure and properties of nanocrystalline Co–W alloy coatings. J. Alloys Compd. 557, 228–238. https://doi.org/10.1016/j.jallcom.2013.01.003.
Subramania, A., Sathiya Priya, A.R., Muralidharan, V.S., 2007. Electrocatalytic cobalt–molybdenum alloy deposits. Int. J. Hydrogen Energy 32, 2843–2847. https:// doi.org/10.1016/j.ijhydene.2006.12.027.
Tait, W.S., 1994. An Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists. PairODocs Publications. https://books.google.com/books? id=dZzyLQAACAAJ.
Tsai, W.-C., Wan, C.-C., Wang, Y.-Y., 2002. Mechanism of copper electrodeposition by pulse current and its relation to current efficiency. J. Appl. Electrochem. 32, 1371–1378. https://doi.org/10.1023/A:1022649314480.
Tsyntsaru, N., Cesiulis, H., Donten, M., Sort, J., Pellicer, E., Podlaha-Murphy, E.J., 2012a. Modern trends in tungsten alloys electrodeposition with iron group metals. Surf. Eng. Appl. Electrochem. 48, 491–520. https://doi.org/10.3103/ S1068375512060038.
Tsyntsaru, N., Cesiulis, H., Budreika, A., Ye, X., Juskenas, R., Celis, J.P., 2012b. The effect of electrodeposition conditions and post-annealing on nanostructure of Co–W coatings. Surf. Coatings Technol. 206, 4262–4269. https://doi.org/10.1016/j.surfcoat.2012.04.036.
Tsyntsaru, N., Cesiulis, H., Pellicer, E., Celis, J.P., Sort, J., 2013. Structural, magnetic, and mechanical properties of electrodeposited cobalt–tungsten alloys: intrinsic and extrinsic interdependencies. Electrochim. Acta 104, 94–103. https://doi.org/ 10.1016/j.electacta.2013.04.022.
V Blagoveshchenskiy, Y., V Isayeva, N., V Blagoveshchenskaya, N., Melnik, Y.I., Chuvildeyev, V.N., V Nokhrin, A., V Sakharov, N., Boldin, M.S., Smirnov, Y.S., V Shotin, S., V Levinsky, Y., Voldman, G.M., 2015. Methods of compacting nanostructured tungsten–cobalt alloys from Nanopowders obtained by plasma chemical synthesis. Inorg. Mater. Appl. Res. 6, 415–426. https://doi.org/10.1134/ S2075113315050032.
Danil’chuk, V., Silkin, S.A., V Gotelyak, A., Buravets, V.A., Mitina, T.F., Dikusar, A.I., 2018. The mechanical properties and rate of electrodeposition of Co− W alloys from a Boron− Gluconate bath: impact of anodic processes. Russ. J. Electrochem. 54, 930–936. https://doi.org/10.1134/S1023193518130116.
V Pervikov, A., Krinitcyn, M.G., Glazkova, E.A., Rodkevich, N.G., Lerner, M.I., 2022. Synthesis of tungsten carbide from bimodal tungsten powder produced by electrical explosion of wire. Int. J. Refract. Met. Hard Mater. 103, 105733 https://doi.org/ 10.1016/j.ijrmhm.2021.105733.
Vernhes, L., Azzi, M., Klemberg-Sapieha, J.E., 2013. Alternatives for hard chromium plating: nanostructured coatings for severe-service valves. Mater. Chem. Phys. 140, 522–528. https://doi.org/10.1016/j.matchemphys.2013.03.065.
Wang, L., Gao, Y., Xu, T., Xue, Q., 2006. A comparative study on the tribological behavior of nanocrystalline nickel and cobalt coatings correlated with grain size and phase structure. Mater. Chem. Phys. 99, 96–103. https://doi.org/10.1016/j.matchemphys.2005.10.014.
Wasekar, N.P., Hebalkar, N., Jyothirmayi, A., Lavakumar, B., Ramakrishna, M., Sundararajan, G., 2020. Influence of pulse parameters on the mechanical properties and electrochemical corrosion behavior of electrodeposited Ni-W alloy coatings with high tungsten content. Corros. Sci. 165, 108409 https://doi.org/10.1016/j.corsci.2019.108409.
Wei, G., Ge, H., Zhu, X., Wu, Q., Yu, J., Wang, B., 2007. Effect of organic additives on characterization of electrodeposited Co-W thin films. Appl. Surf. Sci. 253, 7461–7466. https://doi.org/10.1016/j.apsusc.2007.03.045.
Weston, D.P., Harris, S.J., Shipway, P.H., Weston, N.J., Yap, G.N., 2010. Establishing relationships between bath chemistry, electrodeposition and microstructure of Co–W alloy coatings produced from a gluconate bath. Electrochim. Acta 55, 5695–5708.https://doi.org/10.1016/j.electacta.2010.05.005.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).



































