Corrosion, or the deterioration of a metal surface through oxidation, has always been one of the most harmful and expensive technical challenges within the finishing industry.
 Simone SanseverinatiWithout taking the proper preventative measures and considering a variety of contributing factors. Luckily, there are many resources today to combat corrosion on any metal surface.
Simone SanseverinatiWithout taking the proper preventative measures and considering a variety of contributing factors. Luckily, there are many resources today to combat corrosion on any metal surface.
In order to properly select a treatment system for corrosion, the causes must first be understood. The most notable consequences come from environmental conditions, spillage, storage, and pollution.
The effects can also be exacerbated by design and construction techniques that use dissimilar materials. Considering these issues when engineering any system with metal surfaces can mitigate at least some of the inevitable problems.
Noble Metals
 The noble metals are the platinum group metals, plus silver and gold. Some scientists include copper, rhenium, and mercury.In chemistry, noble metals are metallic elements that resist oxidation, even at high temperatures. The term “noble metal” dates back to at least the late 14th century, describing metallic elements that are fairly unreactive to oxygen, much like noble gases are nearly inert nonmetals. The opposite of a noble metal is a base metal.
The noble metals are the platinum group metals, plus silver and gold. Some scientists include copper, rhenium, and mercury.In chemistry, noble metals are metallic elements that resist oxidation, even at high temperatures. The term “noble metal” dates back to at least the late 14th century, describing metallic elements that are fairly unreactive to oxygen, much like noble gases are nearly inert nonmetals. The opposite of a noble metal is a base metal.
But, the definition of noble metals and list of elements included in the group varies somewhat between disciplines. For example, in physics, a noble metal is a metallic element with filled electron d-bands.
The noble metals are the platinum group metals, plus silver and gold. Some scientists include copper, rhenium, and mercury.
The noble metals are the six platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, platinum), plus silver and gold. Some chemists include rhenium and mercury. Although its properties are not known fully, roentgenium may be a radioactive noble metal. Because of its position on the periodic table relative to silver and gold, some lists include copper.
Noble metals list
| Atomic Number | Element Name | Element Symbol |
| 29 | Copper | Cu |
| 44 | Ruthenium | Ru |
| 45 | Rhodium | Rh |
| 46 | Palladium | Pd |
| 47 | Silver | Ag |
| 75 | Rhenium | Re |
| 76 | Osmium | Os |
| 77 | Iridium | Ir |
| 78 | Platinum | Pt |
| 79 | Gold | Au |
| 80 | Mercury | Hg |
According to the definition of physics, the only noble metals are copper, silver, and gold.
Noble Metal Properties
The noble metals share several common properties:
- Resist oxidation: Resistance to oxidation is the defining characteristic of a noble metal. These elements can form oxides. For example, silver tarnishes, and copper oxidizes to form verdigris. However, noble metal oxides readily decompose when exposed to heat. Similarly, noble metals resist oxidation in moist air and hot water.
- Resist corrosion: Noble metals resist attack by acids and other chemicals, but the level of resistance varies according to the element. For example, palladium and silver dissolve in nitric acid, but platinum and gold resist acids except for aqua regia. Some metals that resist corrosion are not noble metals, such as titanium, niobium, and tantalum.
- High electrical conductivity: In general, metals are good conductors of heat and electricity. But, the noble metals are among the best electrical conductors. Their corrosion resistance makes them popular choices for electrodes, contacts, and wires.
- Catalytic activity: The partially filled d-subshells of noble metals (under the chemistry definition) make these elements excellent catalysts.
- Electron affinity: The noble metals have high electron affinity values.
- Siderophilic: The noble metals are siderophiles (“iron lovers”). They readily dissolve in molten iron or iron solutions. As a consequence, these elements likely accumulate in the Earth’s core.
- Native elements: The six platinum group metals, copper, silver, and gold, are the only metals that occur in relatively pure form in nature (native).
Noble Metal Uses
 Understanding dissimilar metals.Noble metals are found in coinage, jewelry, medicine, and electronics, as protective coatings, and in chemistry as catalysts. Platinum, gold, silver, and palladium are bullion metals that are used in coins and jewelry. Noble metals are often plated over base metals to protect them and add value. Copper, gold, and silver are used in medicine as antimicrobial agents.
Understanding dissimilar metals.Noble metals are found in coinage, jewelry, medicine, and electronics, as protective coatings, and in chemistry as catalysts. Platinum, gold, silver, and palladium are bullion metals that are used in coins and jewelry. Noble metals are often plated over base metals to protect them and add value. Copper, gold, and silver are used in medicine as antimicrobial agents.
Copper, gold, and silver are used in wires, contacts, and electrodes. Platinum, palladium, rhodium, ruthenium, and iridium are important catalysts. Ruthenium and iridium are hard metals that strengthen alloys and are used in small machine parts, pen nibs, and spark plugs.
Zinc Flakes Basecoats
The main task of basecoats is to actively protect the coated steel surface against corrosion. This protective effect is based on the sacrificial property of the zinc contained in all basecoats.
The less noble a metal is, the greater its tendency to corrode ("rust"). Zinc is very base compared to iron and steel. Therefore, it will corrode first in unfavorable environmental conditions.
Corrosion is an electrochemical process in which the metal involved in the reaction oxidizes.
If the oxidizing metal is electrically connected to another metal, the electrons released during oxidation flow from the base metal (here zinc) into the more noble metal (here steel).
Hence, the terms "sacrificial anode" (zinc corrodes) and "cathodic corrosion protection" (steel is protected). If the coating is damaged, the zinc sacrifices itself to protect the steel surface.
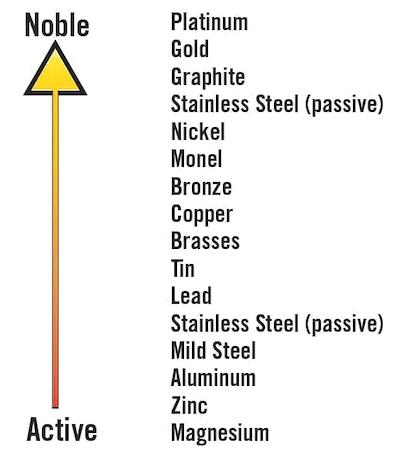
Metals come with different inherent properties. One such trait, their nobility, plays an important role in determining how metals are ranked on the galvanic scale. Here, nobility refers to a metal's resistance to corrosion. For example, noble metals that are higher up the galvanic scale retain their electrons better compared to base metals at the lower end of the scale when placed within the same environment.
 A base metal such as aluminum loses more of its electrons than a noble metal such as stainless steel. Hence, when two different or dissimilar metals from opposite ends of the galvanic scale are in contact and are subjected to a corrosive environment, electrons are transferred from the base metal to the noble metal. The transfer of electrons (also expressible as the current flow) accelerates the base metal's corrosion rate and further reduces the corrosion rate of the noble metal.
A base metal such as aluminum loses more of its electrons than a noble metal such as stainless steel. Hence, when two different or dissimilar metals from opposite ends of the galvanic scale are in contact and are subjected to a corrosive environment, electrons are transferred from the base metal to the noble metal. The transfer of electrons (also expressible as the current flow) accelerates the base metal's corrosion rate and further reduces the corrosion rate of the noble metal.
For example, using aluminum and stainless steel to build structures within an aircraft will result in dissimilar corrosion rates. The corrosion rate of aluminum will increase while that of stainless steel slows. This imbalance is accelerated in highly corrosive environments with excessive moisture and changing temperatures and will cause the aircraft's structure to crumble and fail if left unattended.
Separating Dissimilar Metals
In theory, limiting the adverse effects of dissimilar metals coming into contact with one another may seem like an easy task. Utilizing all the same metal or galvanically similar metals should solve the problem. However, in the real world, some dissimilar metal use is unavoidable thanks to the properties they bring to the table. In such a scenario, four major preventive measures can be applied to separate dissimilar metals and slow corrosion.
- Using non-absorbent insulating materials — When dissimilar metals must be placed side-by-side, applying a two-sided polyurethane tape between both metals reduces the effects of galvanic corrosion. The use of a nonabsorbent insulating material slows the transfer of electrons and minimizes the corrosion rate by denying electrons an electrolyte through which to travel. Nonabsorbent tapes also protect the metal surfaces from non-galvanic corrosion modes. Keeping out moisture and inhibiting the loss of electrons will reduce corrosive reactions.
- Apply protective coatings or prime the metals — Protective coatings and paints are known for their ability to insulate metals against corrosion. Emphasis should be placed on painting or priming joints that consist of dissimilar metals. Pay special attention to properly coating the more galvanically active metal in the configuration.
- Utilize nonmetal washers and gaskets — Threaded fittings and fasteners are often made of metals different from the parts they secure. Eliminating them or physically separating them from contact with the metal part can reduce corrosion rates. Plastic washers and gaskets are among the simplest tools for this task. These simple protective components separate dissimilar metal surfaces from corrosive agents and galvanic reactions.
- Use tape instead of caulk — Maintenance and manufacturing service providers regularly utilize caulk to join metals or reject moisture. Over time, however, caulk is likely to get squeezed out of dissimilar metal joints, allowing both metals to contact one another or water to creep in. In place of caulk, polyurethane tape is the best option for joints. It sticks to the surfaces themselves and does not get squeezed out. Quality tape is also easy to remove when further maintenance work is required.
Stop Corrosion When Using Dissimilar Metals
Utilizing dissimilar metals is a notorious cause of corrosion within the aviation, naval, heavy trucking, and manufacturing industries. All that equipment also tends to wind up in environments with diverse corrosive agents.
Proper use of durable sealants, coatings, gaskets, and tapes can limit the damaging effects of dissimilar metals and keep moisture out.
Corrosion-Prevention Considerations
In order to understand which corrosion prevention solution you need, it is important to discuss the most notable contributing causes.
The most effective way to deter corrosion is to implement a specific prevention plan combining the most suitable pretreatments, primers, and paints while taking into account the above variables. Ideal corrosion solutions optimize affordability and mitigate exposure to hazardous materials.
In addition to acting as sealants, products like pretreatments, primers, and paints further combat corrosion by resisting condensation and regulating temperature.
Simone Sanseverinati is the Business Development Manager for UV Coatings at Dörken Coatings. Visit https://www.doerken.com.



































