Most surface finishers will review P&L statements each month to gauge their business health, but for Ross Peterson at ProPlate, there are other metrics he studies.
 Ross PetersonPeterson is the Chief Business Development Officer of ProPlate, an Anoka, Minnesota, company that finishes specialty items for the medical and surgical industry. Peterson often receives information from OEMs, doctors, and hospitals on how the medical parts ProPlate helped develop have helped patients.
Ross PetersonPeterson is the Chief Business Development Officer of ProPlate, an Anoka, Minnesota, company that finishes specialty items for the medical and surgical industry. Peterson often receives information from OEMs, doctors, and hospitals on how the medical parts ProPlate helped develop have helped patients.
Case in point: ProPlate spent over a year developing a process to finish neodymium iron boron magnets, which can be up to 10 times stronger than the best ceramic magnets. Surgeons were hoping to implant some of the NdFeB magnets into newborns to help them with serious medical conditions that risked their ability to survive.
Due to the base magnet material's porosity and the magnets' reactivity to acidic and basic chemistries, ProPlate faced a difficult challenge in designing a plating process for the magnets. Still, after a long R&D process, they met the requirements.
Photographs Tell the Story of Plating’s Impact
 ProPlate spent over a year developing a process to finish neodymium iron boron magnets, which can be up to 10 times stronger than the best ceramic magnets. And that is when the photographs started arriving at ProPlate’s headquarters.
ProPlate spent over a year developing a process to finish neodymium iron boron magnets, which can be up to 10 times stronger than the best ceramic magnets. And that is when the photographs started arriving at ProPlate’s headquarters.
“There's no other good option for these babies that are born with this condition,” Peterson says. “And one of the most rewarding things is when we were done with that project, we were sent some pictures from that university of the babies and the families that used that device successfully. It was better than any form of compensation I've ever received.”
ProPlate specializes in the barrel and rack electroplating processes, focusing on precise, selective plating for precious and semi-precious metals. It offers finishes in gold, rhodium, platinum, palladium, silver, nickel, palladium-nickel, and copper plating solutions. ProPlate predominantly serves the medical, dental, semiconductor, electronics, energy, technology, aerospace, and government industries.
Their primary focus is working with medical device engineers and designers — from start-ups to the largest OEMs in the world — to develop innovations through unique metal coating technologies that drive commercial success and improve patient outcomes.
That sense of pride in helping doctors, surgeons, and those in the medical industry has helped grow the company since it was founded 35 years ago.
“We always go back to the patient side of it; that's been some of the most rewarding work to know the impact we are having through improving people’s lives.”
“We get to live in that true sense of an R&D world where we're inventing new processes,” Peterson says. “Sometimes we're just taking concepts of different processes and combining them for a new outcome, often resulting in a new and novel processing technique. Being involved in some of those projects has been a fun path.”
It is especially rewarding when they see the results of their work, whether it is saving newborns or helping medical companies overcome design issues that were considered undoable.
“It’s great to see from start to finish what a concept can start as and then be something that gets used in a medical device,” Peterson says. “We always go back to the patient side of it; that's been some of the most rewarding work to know the impact we are having through improving people’s lives.”
Moving into a Facility Three Times the Previous Space
 ProPlate specializes in the barrel and rack electroplating processes, focusing on precise, selective plating for precious and semi-precious metals. The growth in their business over the last decade has caused ProPlate’s owners—Craig and Suzanne Ingalls, Peterson’s stepfather and mother—to consider the need for more space. This resulted in the company's significant expansion from a 12,400-square-foot facility to a 38,500-square-foot space.
ProPlate specializes in the barrel and rack electroplating processes, focusing on precise, selective plating for precious and semi-precious metals. The growth in their business over the last decade has caused ProPlate’s owners—Craig and Suzanne Ingalls, Peterson’s stepfather and mother—to consider the need for more space. This resulted in the company's significant expansion from a 12,400-square-foot facility to a 38,500-square-foot space.
Peterson says this growth enhances their capabilities and marks a pivotal moment in their journey of innovation, one that is based on its commitment to improving lives through cutting-edge innovations and exceptional services.
It has been challenging to go to a location almost three times its previous site—albeit only about five miles from its original location—in mid-July. Still, it hasn’t affected their work, research, or processes for its customers.
“The move is about as least complex as it can be in terms of distance from the original facility, which isn't to say that it's not a big lift,” Peterson says. “We are creating all new plating lines and equipment.”
The shift to the new facility gives them about five times the office space they had before. Peterson says it seems to be one of their biggest growing headcounts as they add more engineering staff and sales, marketing, and admin support positions.
The production floor space is three times the size as before, as are their R&D area and new product development centers. The move will also give them room to grow, as about 7,000 square feet in the production space have not been tagged for anything yet.
“There are many projects in the pipeline that will be a good fit for it,” Peterson says. “With the long sales cycles of medical devices, those projects usually come into the pipeline two to four years from when we start them. So we do have time to plan around for that.”
ProPlate will keep the other facility open as a redundancy since the medical device industry takes risk very seriously. Having a disaster recovery plan — including multiple manufacturing facilities — is crucial to securing programs with the industry's top OEMs and ensuring supply continuity.
 The company underwent a significant expansion from a 12,400-square-foot facility to a 38,500-square-foot space.
The company underwent a significant expansion from a 12,400-square-foot facility to a 38,500-square-foot space.
Fully Climate Controlled Facility Adds to Comfort and Quality
 ProPlate’s owners Craig and Suzanne Ingalls in the company’s new 38,500-square-foot space.Another highlight of the new facility is the real-time bath monitoring capabilities, and accommodation has been built into the design for further processing and chemistry management automation.
ProPlate’s owners Craig and Suzanne Ingalls in the company’s new 38,500-square-foot space.Another highlight of the new facility is the real-time bath monitoring capabilities, and accommodation has been built into the design for further processing and chemistry management automation.
There is also 90,000 sq. ft. of additional land to expand their footprint at the new facility. Peterson also says one of the highlights of the new facility is that it will have a fully air-conditioned production floor.
“In the past, we didn't have climate control,” he says. “Not only is this improvement good for operators and employees, it's also beneficial for the process. We can now control humidity and temperature in the summer and winter, which will help maintain that control of the process.”
ProPlate is also installing a newer water circulation system that will recirculate the water in its rinse tanks and substantially reduce their usage from 15,000 gallons per day to less than 200 gallons, saving nearly 4 million gallons of water annually. Peterson says that will keep the tanks even cleaner, as they filter the incoming water rather than just the outgoing waste stream to the discharge.
“That's going to help control our quality even to a higher degree,” he says. “And everything will be DI-water based versus just some of our baths.”
“It takes a long time to work your way into it. You have to establish your reputation, and you have to sharpen your tools. You must present yourself differently than a plating company traditionally looks like.”
ProPlate began searching for new space about three years ago. Once they found it, they spent a year planning the floor plan and building out the facility while continuing operations in their old space. They began moving into the facility in July, Peterson says, and they will be fully operational in a few months.
Part of the move-in timeline is because ProPlate works in the medical device industry, and some of its customers require the processes to be re-qualified on the new equipment and the baths used to finish parts.
“It's a clone in that the new facility uses the same chemistries, the same bath sizes, and the equipment is the equivalent,” he says. “But we do have to work with each customer to ensure that we do it correctly if we need to go through re-qualifications. We will have operations working at the old and new facilities, so we can transition seamlessly.”
A Shift to Medical Finishes Starts Big Growth Phase
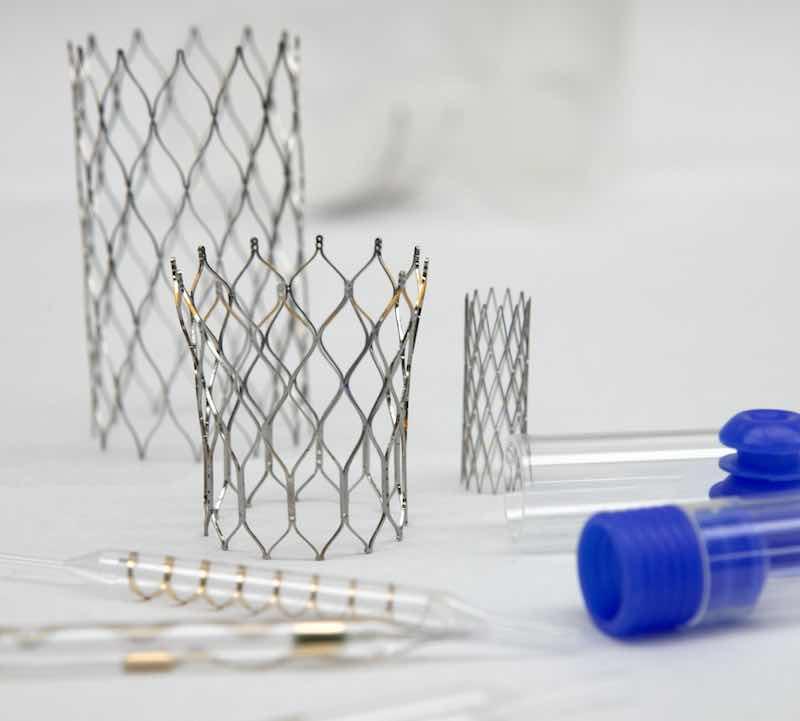 ProPlate coats medical stents and balloons that are crucial for life-saving medical procedures.Peterson says ProPlate started shifting its focus to medical finishes about 15 years ago when it saw a shortage of shops that could handle the industry's complex research and specialized processes.
ProPlate coats medical stents and balloons that are crucial for life-saving medical procedures.Peterson says ProPlate started shifting its focus to medical finishes about 15 years ago when it saw a shortage of shops that could handle the industry's complex research and specialized processes.
“It takes a long time to work your way into it,” he says of the medical device industry. “You have to establish your reputation, and you have to sharpen your tools. You must present yourself differently than a plating company traditionally looks like.”
He and Ingalls found that the medical device world knows little about plating but much about developing products. That led ProPlate to change its marketing to be more product-based and saw the development of its proprietary Vizi-Band radiopaque marker coating innovation. This metal coating process atomically bonds directly to a catheter, guidewire, stent, or similar component.
Vizi-Band’s highly radiopaque and biocompatible metal coating — which Peterson says is selective plating of gold or platinum — can be selectively applied to any section of the desired component, with endless options for customization depending on the application and intended use. It also replaced traditional marker bands and ring electrodes with performance and potential cost advantages.
ProPlate has also developed its’ Meta-Poly proprietary vacuum deposition process, which atomically bonds metal directly to a polymer substrate without using toxic chromic acid. Peterson says this is a PVD process for metalizing circuitry onto thin-walled, flexible balloons or other polymers for medical devices.
“Rather than just going to a trade show and saying, ‘We can offer plating,’ we showed that we offered maybe more value and better features and benefits than the traditional ones. There are plating opportunities in the medical device industry with more traditional ASTM standard specs, but we strive to be innovators.”
“The applications range from surgical devices to implantables,” Peterson says. “And anything from a neurosurgery — like electrical surgical forceps requiring silver plating for its thermal and electrical conductivity — to the marker bands and electrodes in catheter devices.”
The radiopaque marker bands and the catheter devices took their selective plating capabilities—a very precise CNC control process where they apply a liquid polymer to a component—and then they can remove very precisely the areas on that component that they want to expose for plating. They take the parts through the plating process, after which they chemically strip away the liquid polymer.
ProPlate usually plates platinum and gold for its density. Under X-ray or fluoroscopy, it shows up very brightly so that the physician can see exactly where that component is and even how it might be oriented.
Finding Additional Plating-On-Polymers Applications
 The production floor space is three times the size as before, as are their R&D area and new product development centers. ProPlate began to find additional applications in the plating-on-polymers sector, and they explored that process for specific balloon applications. In these applications, gold can be deposited directly onto a balloon used in a catheter device for ablation procedures, sensing, or stimulation.
The production floor space is three times the size as before, as are their R&D area and new product development centers. ProPlate began to find additional applications in the plating-on-polymers sector, and they explored that process for specific balloon applications. In these applications, gold can be deposited directly onto a balloon used in a catheter device for ablation procedures, sensing, or stimulation.
“Whatever electrical circuitry might be used for those applications — sensing, stimulation, mapping, and ablation — we can apply that,” Peterson says.
The company has won several awards from the medical coatings industry and accolades for its partnership in helping bring these surgical items to market when manufacturers were struggling.
Peterson says that over the past decade or more, ProPlate has found its new calling as an expert in the plating industry for medical devices and procedures and in ways to innovate these products with manufacturers.
“It was a shift in our thinking, and it was just how we marketed ourselves,” Peterson says. “Rather than just going to a trade show and saying, ‘We can offer plating,’ we showed that we offered maybe more value and better features and benefits than the traditional ones. There are plating opportunities in the medical device industry with more traditional ASTM standard specs, but we strive to be innovators.”
Visit www.proplate.com.




































