Conventional electroplating processes for magnesium and its alloys have been in existence for many years.
 Rich BellemareThese processes have entailed the use of concentrated hexavalent chromium solutions, hydrofluoric acid solutions and electrolytic cyanide-containing plating solutions. Subsequent plating with electroless nickel has proven problematic due to the extreme instability of the electroless nickel solutions in contact with magnesium.
Rich BellemareThese processes have entailed the use of concentrated hexavalent chromium solutions, hydrofluoric acid solutions and electrolytic cyanide-containing plating solutions. Subsequent plating with electroless nickel has proven problematic due to the extreme instability of the electroless nickel solutions in contact with magnesium.
This paper describes a new RoHS-compliant process for the electroless nickel coating of magnesium which is stable and contains no hexavalent chromium or cyanide.
Introduction
Technological trends in the computer, electronics and telecommunications industries continue to drive miniaturization, reduction in the number of components and increased functionality of both the components within a device and the device itself. Consumers now expect electronic products to provide multifunctional capabilities in one device, such as telephone, camera, text messaging, personal organizer, PC software, MP3 player, alarm, GPS, etc.
Miniaturization also requires that the various components be combined within a single enclosure. Many of the component parts have been consolidated into single, multifunctional parts in order to:
- Keep manufacturing costs down
- Simplify the assembly process
- Further reduce size
- Allow greater design freedom for the final product
Previously, for example, the function of structural housings might have been solely to protect and support the internal parts of a device. Today, that same housing is a fraction of the size and cross-section as the original, but still needs to support the internal parts with the same degree of protection while also serving as an EMI/RFI shield and heat sink.
This change in part design and functionality has resulted in more stringent requirements for materials of construction in terms of strength, weight and manufacturability. The required properties for these new designs include:
- High stiffness in thin cross-sections
- High strength-to-weight ratio
- High dimensional stability
- High thermal and electrical conductivity
- Ease of manufacturing
Magnesium is the primary material to meet these stringent demands in more and more applications.
Properties of Magnesium
Magnesium (Mg) is the eighth most abundant element in the Earth’s crust. It is much lighter than steel, copper and even aluminum, rivaling the density of plastic. Magnesium has a high strength-to-weight ratio, allowing the design of very thin and light parts with high strength. Dimensional stability, combined with ease of forming and casting, makes it suitable for the high volume production of intricate parts. Table 1 summarizes some of the properties of magnesium.
Table 1 Properties of magnesium
| Property | Advantage | |
| Density | 1.74 | 67% of aluminum; 23% of steel; 27% of zinc; 165% of plastic |
| High Tensile Strength | 280 MPa | Higher strength-to-weight ratio than plastic |
| High Thermal Conductivity | 72 W/Mk | Better heat dissipation than plastic |
| High Electrical Conductivity | 6.6 MS/m | Better EMI/RFI shield than plastic |
| High Dimensional Stability | Maintain tight tolerances | |
| Recyclability | Easy to recycle | |
| Abundance | Very abundant |
Although magnesium has many desirable physical properties, it is also easily attacked by corrosive materials because it is one of the most electrochemically active materials. Until recently, this property has limited the use of magnesium in applications where corrosive environments are encountered. Usually this issue is remedied by a coating to protect it from the outside environment.
A number of coating technologies exist for the protection of magnesium. Each technology has benefits and has found acceptance in various industrial applications. Some of these technologies are outlined in Table 2.
The ultimate choice of coating depends upon the final performance requirements of the particular piece and the cost restrictions imposed upon the project.
Magnesium has been plated with electrolytic coatings since the 1950s. One of the pioneers in magnesium plating was The Dow Metal Products Company, with the development of a series of pretreatments and plating systems for various alloys. These systems generally consisted of hexavalent chromium-based etches, hydrofluoric acid based activators, zincate and cyanide copper strikes prior to additional topcoats. When tightly maintained and controlled, these systems provided very viable systems for the successful plating of magnesium.
In today’s environmentally sensitive world however, it is increasingly difficult to set up new lines utilizing these types of materials, and RoHS legislation has restricted the use of certain materials in the plating processes. Environmentally conscious companies are banning the use of hexavalent chromium in finishing processes, and many countries and municipalities are making it virtually impossible to set up lines containing cyanide chemistries. This necessitates development work on new processes for the pretreatment and coating of magnesium and its alloys.
Magnesium Alloy
The most common magnesium alloy currently used in electronics and telecommunications is AZ91D because of its superior castability and good mechanical properties. Alloy AZ91D contains 9 wt% aluminum and 1 wt% zinc, and in the as-cast condition, has a surface that is very heterogeneous, due to the formation of Mg17Al12 intermetallic compounds during the melting, casting and cooling processes. These intermetallic compounds can set up strong galvanic corrosion cells with magnesium resulting in accelerated corrosion of the magnesium substrate. The SEM image and EDS mapping in Fig. 1 illustrate this heterogeneous distribution of elements.
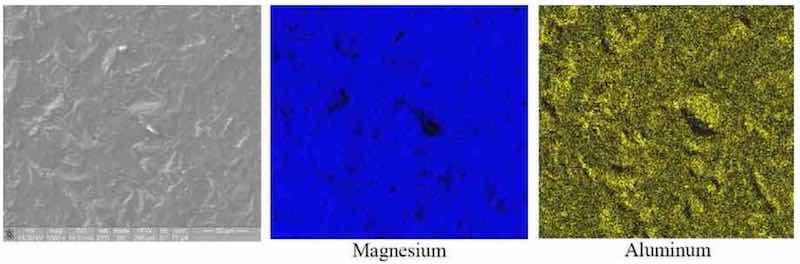
Figure 1—As-Cast AZ91D: SEM Image and EDS mapping for magnesium and aluminum.
Table 2 Magnesium coating technologies
| Technology | Comments |
| Chemical conversion coating | Good corrosion protection; High throughput; Soft coating is not wear-resistant; Coating is non-conductive. |
| Anodizing | Hard coating is wear-resistant; Good corrosion protection; Good throughput; Coating is non-conductive. |
| PVD | Wide variety of deposits available for hardness; decorative; etc; Coverage limited to simple geometries due to line-of-sight application; Limited throughput; High capital costs. |
| Painting | Wide variety of colors; Good corrosion protection; Good throughput; Coating is soft; Coverage limited to simple geometries due to line-of-sight application; Coating is non-conductive. |
| Electroplating | Variety of metal coatings available; Good wear and corrosion resistance; High conductivity; Good throughput; Difficult to plate complex geometries and blind areas; thus reducing corrosion resistance. |
| Direct electroless nickel plating | Coverage of complex geometries; Good hardness and wear resistance; High conductivity; High throughput; Can be top-coated. |
Plating Process
The four important processes key to obtaining optimum adhesion and corrosion protection for this alloy are:
- Pretreatment process capable of cleaning, etching and removing surface alloying elements to render the surface of the magnesium as pure as possible,
- Conversion coating process which seals and prevents oxidation of the magnesium surface,
- Electroless nickel process with a very high tolerance to magnesium exposure and
- Surface drying and annealing process for 1 hr at 375°F (190°C) to develop full adhesion.
Pretreatment
The first stage of the pretreatment process must remove any oils and soils, which have settled onto the surface of the parts through handling and packaging prior to delivery to the plating line. Typically, high detergency alkaline soak cleaners are used. Magnesium is resistant to attack by alkaline solutions and is, therefore, relatively unaffected by exposure to these cleaners. Failure to remove surface oils completely results in uneven etching and activation of the surface during the later pickling processes. This could result in local areas of poor adhesion as well as the presence of corrosion cells and high deposit porosity, which ultimately affect the product performance.
The next steps in the preparation of magnesium for plating involve exposing and removing the surface alloying elements while depositing a thin protective film onto the magnesium. This prevents formation of a detrimental oxide film. Conventional etches used to expose the components of the magnesium alloy had historically contained high concentrations of hexavalent chromium in the form of chromic acid and nitric acid blends. Fortunately, proprietary RoHS-compliant etches of today perform a similar task without the use of hexavalent chromium.
The condition of the surface after etching with a RoHS-compliant system can be seen in Fig. 2. The surface of the magnesium has been micro-roughened by the etch and enriched in the alloying elements aluminum and zinc. These elements exist as a dark smut on the surface of the magnesium.
After etching, the AZ91D is then immersed into an activator solution that removes the smut generated in the etching process. In general, conventional and proprietary activators contain high concentrations of fluoride that effectively “de-smut” the magnesium surface, leaving it clean and relatively pure. The high fluoride containing solutions also deposit a thin protective magnesium fluoride coating which slows the formation of unacceptable oxide films (Fig. 3).
Conversion Coating
After the surface of the magnesium has been cleaned, etched and de-smutted, a conversion coating is then applied to the magnesium. Similar to a zincate on aluminum, the conversion coating protects the magnesium surface from forming an oxide film, which would prevent good adhesion of subsequent coatings. Conventional processes utilize a zincate specifically designed for magnesium followed by a cyanide copper strike that leaves the zincate film intact. The cyanide copper deposit then acts as a protective barrier between the magnesium substrate and the subsequent electroless nickel layer.
In the direct plating, without the intermediate cyanide copper strike, immersion of the zincated magnesium into the electroless nickel solution typically removes the zincate film, exposing the magnesium to potential attack by the components of the electroless nickel solution. The electroless nickel solution is also exposed to the potential reactions with bare magnesium. This can result in inconsistent adhesion of the deposit to the magnesium and instability of the electroless nickel bath. The current technology incorporates the application of a conversion coating specifically designed to withstand immersion in the electroless nickel solution and remain as a barrier layer between the magnesium and electroless nickel (Fig. 4).
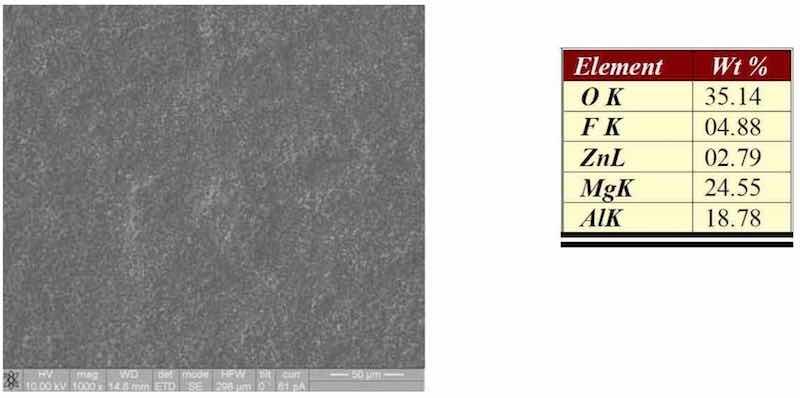
Figure 2—SEM/EDS of AZ91D after etching.

Figure 3—SEM/EDS of AZ91D after activation.
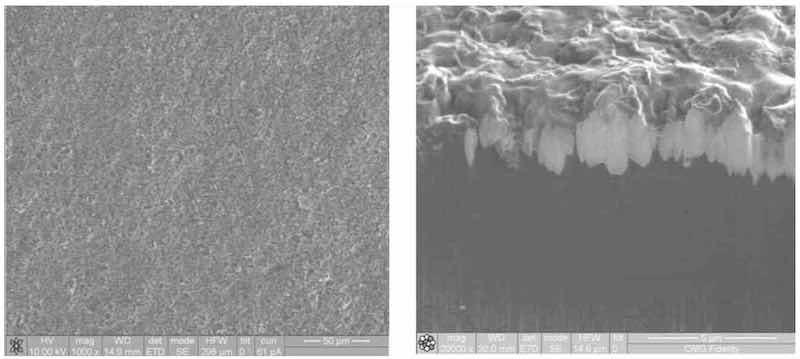
Figure 4—Surface and cross-section of magnesium with conversion coating.
Electroless nickel plating
After the conversion coating, the magnesium substrate is immersed in the electroless nickel solution for deposition of the primary protective layer. The most common systems have used conventional electroless nickel systems modified with the addition of a fluoride source to enhance the performance of the solutions.
As bare magnesium is placed into a conventional electroless nickel solution, it is quickly attacked and corroded by the components of the electroless nickel bath. Upon dissolution, the magnesium quickly reacts with orthophosphite, the by-product of hypophosphite oxidation, to form insoluble precipitates. These precipitates cause many issues including:
- Formation of excess porosity in the plated deposit that contributes to poor corrosion performance and adhesion
- Rapid consumption of the stabilizers in the electroless nickel solution resulting in bath instability
- Plate-out
- Shortened bath life
- Inconsistent appearance
It is understandable that platers of magnesium routinely get only one to two metal turnovers from their electroless nickel baths.
To overcome premature precipitate formation, it is critically important that the electroless nickel solution is designed for magnesium exposure, whether plating directly or indirectly on magnesium. The electroless nickel solution must also be capable of quickly initiating, sealing and building a deposit with high integrity over the substrate surface (Fig. 5).
Top Coats
After the initial electroless nickel deposit has been established, many options exist to meet the variety of industrial requirements. Table 3 outlines a number of these options.
The main advantage of plating electroless nickel directly onto magnesium prior to top coating is that the electroless nickel covers all surfaces and protects the magnesium from attack by other plating systems. This is especially true for complex shaped parts with deep bosses and blind holes that electrolytic and paint systems cannot reach.
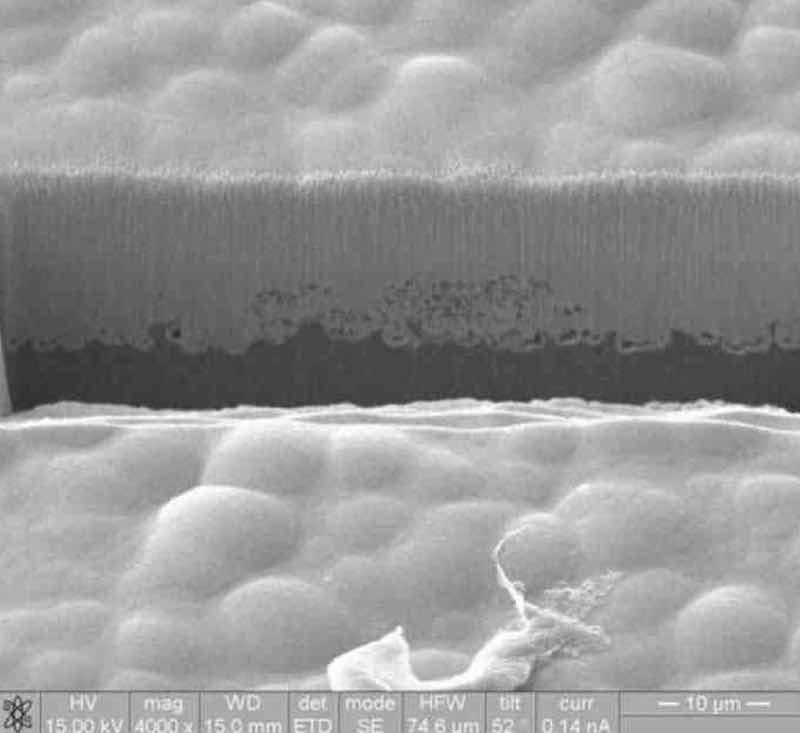
Figure 5—Focused ion beam (FIB) image of the electroless nickel deposit.
Performance
Direct plating of magnesium with electroless nickel, either as a stand-alone coating or in combination with others, has the demonstrated ability to meet the adhesion, appearance and corrosion standards currently in place. There are, however, key factors unrelated to the plating process that can greatly influence the quality of the plated part.
The most important is the quality of the cast magnesium part. In the case of AZ91D, which is a highly suitable alloy for plating, a caster can render parts out of this alloy useless for plating simply by the way the parts are cast. Figure 6 depicts several casting defects, which are highly detrimental to plating.
The main casting defects that make a magnesium part difficult to plate are (1) porosity, (2) incomplete filling of the mold and (3) flash. Plating solutions are unable to penetrate and adequately coat surfaces with defects from incomplete mold filling or high porosity, and the result is a discontinuous deposit with open pathways for corrosive media to enter and attack the magnesium substrate directly. In the case of flash, although completely coated with electroless nickel, it remains quite fragile and can easily break off during handling. This results in exposure of the magnesium, which is then easily attacked by corrosion, the corrosion rate being enhanced by the galvanic couple formed between the electroless nickel and the magnesium.
Table 3: Post-electroless nickel options
| Option | Application |
| Increased electroless Ni thickness | Increased corrosion resistance |
| Electrolytic Cu/electroless Ni | Increased corrosion resistance |
| Electrolytic copper/electrolytic Ni/Cr | Decorative/corrosion resistance |
| Acid tin | Solderability |
| Immersion gold | Solderability |
| Paint | Decorative/corrosion resistance |
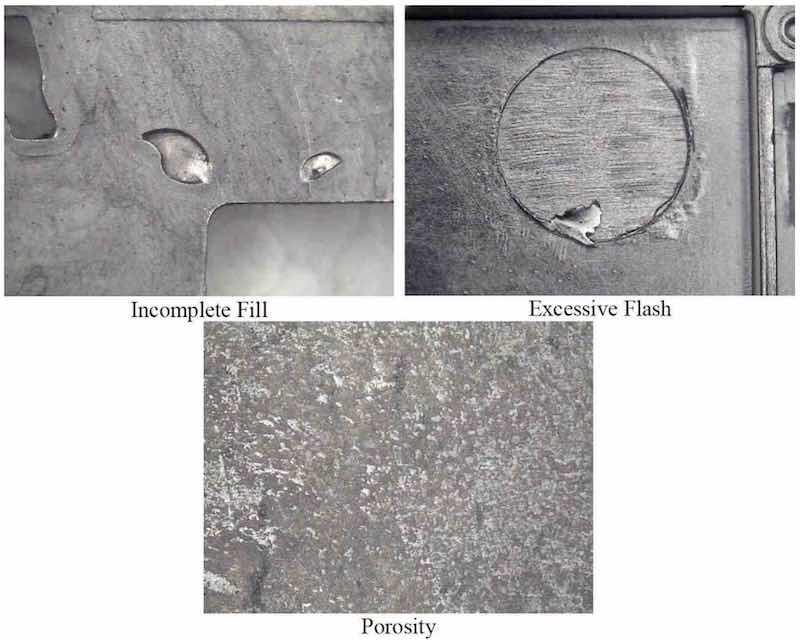
Figure 6—Casting defects.
With well cast parts, good results are obtainable in terms of adhesion, appearance and corrosion resistance. Typical results, though not all-inclusive, are illustrated in Fig. 7 and Table 4.
Conclusions
The advantages of the direct electroless nickel plating over other finishing technologies including electroplating include:
- The ability to completely cover complex geometries, blind holes and deep bosses
- Protection of the magnesium from subsequent plating solutions and corrosive environments
- Development of a hard deposit
- Wear resistance
- Corrosion resistance
- Electrically-conductive deposit for shielding
- The ability to topcoat with numerous materials

Figure 7—Cross-hatch tape test and neutral salt spray (NSS) results.
Table 4 Comprehensive test results
| Testing Done | Results (Specifications) |
| Cross-hatch/tape test with Scotch 610 tape (as plated) | Pass |
| Cross-hatch/tape test with Scotch 610 tape (24 hr NSS) | Pass |
| 4 hr NSS per ASTM B-117; 168 hr humidity (40°C; 93%RH) | Pass (<5% corrosion) |
| 24 hr NSS continuous per ASTM B-117 | Pass (<5% corrosion) |
| 72 hr (3 cycles of 8 hr NSS per ASTM B-117; 16 hr idle) | Pass (<5% corrosion) |
| Top-coated with bright acid copper; bright nickel and trivalent chromium | Pass adhesion |
| 72 hr NSS with EN/Cu/EN | Pass (<1% corrosion) |
| Quench tested 190°C to 25°C | Pass with no adhesion loss |
Direct electroless nickel processes specifically designed for magnesium can consistently and reliably direct plate on AZ91D magnesium alloys. This new system is totally RoHS-compliant and eliminates the use of hexavalent chromium and cyanides (though they can be used should the application require), while providing a stable, reliable electroless nickel plating process. The direct plating system, as stand alone or in combination with other topcoats, provides the designer with multiple alternatives to meet current and future requirements.
References
1. H.E. Friedrich & B.L. Mordike, Eds., Magnesium Technology, Springer-Verlag, Berlin, Heidelberg, New York, 2006.
This paper was originally published in 2010.
About the Author
Rich Bellemare served as Global Technical Manager at OMG Electronic Chemicals, Inc., which is now owned by MacDermid Enthone Industrial Solutions. He is now Global GDAC Director at MacDermid Alpha Electronics Solutions. Bellemare is a graduate of Rensselaer Polytechnic Institute, Troy, New York, where he earned a Bachelor of Science degree in Chemistry.



































