A plating shop manager was expected to troubleshoot each of their 22 processing lines.
 Frank Altmayer“I have read most of the electroplating ‘bibles,’” he wrote to me. “But I sure could use some basic ideas on how to go about troubleshooting a plating process. Can you steer me in the right direction?”
Frank Altmayer“I have read most of the electroplating ‘bibles,’” he wrote to me. “But I sure could use some basic ideas on how to go about troubleshooting a plating process. Can you steer me in the right direction?”
He doesn’t mention which Bibles he had read, but I would hope that one of them is Trouble in Your Tank? by Larry Durney, a book available through AESF. As for help in sorting out the task of troubleshooting, I was willing to give it a shot.
To have a chance of success at troubleshooting any electroplating process, you need to develop a knowledge base and follow basic guidance in detective work. The knowledge base breaks down as follows:
- Types of Defects
- Problems with Base Metals
- Problems with Equipment
- Problems with Solutions
- Problems with Operations
Types of Defects
While there are hundreds of types of defects in electroplating, the most common ones are:
- Poor Adhesion
- Pitting
- Off Color
- Burned Deposit
- Dark Deposits
- Poor Coverage
- Roughness
- Poor Solderability
Let’s look at each of these.
Poor Adhesion: Ferrous Substrates
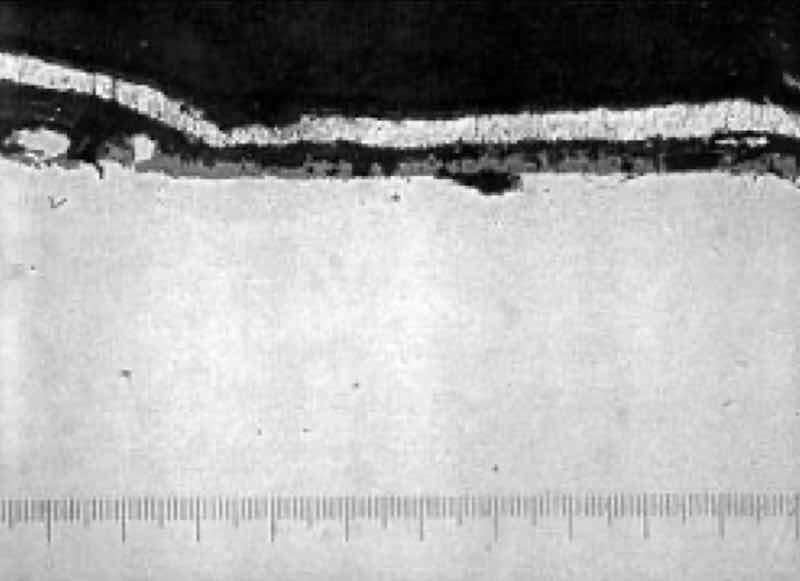 Photomicrograph at 600X, of heat treat scale on zinc plated steel parts, evidencing poor adhesion.From my experience, 90% or more of all problems involving poor adhesion on ferrous substrates (steels, cast iron) have to do with how the part is processed prior to the deposition of the major plated layers. This eliminates about 30%–50% of the process from the initial investigation, but still leaves quite a large amount of detective work to be done. The obvious place to start is at the beginning of the line. Each of the processing steps is intended to take the part one step closer to the point where the surface of the part is in an “active” condition. An active condition is present when the part has metal atoms on the surface that are not bound to oxides, such as rust, or invisible traces of metal oxides, such as heat treat scale, and there are no barriers between the surface atoms of metal and the metal atoms produced by the plating process, such as traces of oil.
Photomicrograph at 600X, of heat treat scale on zinc plated steel parts, evidencing poor adhesion.From my experience, 90% or more of all problems involving poor adhesion on ferrous substrates (steels, cast iron) have to do with how the part is processed prior to the deposition of the major plated layers. This eliminates about 30%–50% of the process from the initial investigation, but still leaves quite a large amount of detective work to be done. The obvious place to start is at the beginning of the line. Each of the processing steps is intended to take the part one step closer to the point where the surface of the part is in an “active” condition. An active condition is present when the part has metal atoms on the surface that are not bound to oxides, such as rust, or invisible traces of metal oxides, such as heat treat scale, and there are no barriers between the surface atoms of metal and the metal atoms produced by the plating process, such as traces of oil.
The best tools here consist of a scrubbing brush with stiff nylon bristles and some pumice (diatomacious earth). Your task is to isolate the problem to a single tank in the processing line. You can start by scrubbing one or more parts on a rack (or some “tagged” parts from a barrel load, after processing step 1, which is usually the soak cleaner). These parts must be marked so that you can recognize them after the entire plating process, so use a scribe or vibrating marker to write your name or suitable graffiti on these parts. Scrub the parts until they are noticeably scratched up, and water sheets from the surface (no water breaks). Now make sure the parts go through the process line exactly under the same conditions as the parts that you did not scrub. If these parts evidence the same adhesion problem as the unscrubbed parts, you may eliminate step 1 from the prime list of suspects. In this case, you now process all the parts through step 1, but scrub the parts after step 2 (which typically is the electrocleaning process). Again, the scrubbed parts must be processed in the exact same manner as the unscrubbed parts, with only process step 2 substituted by scrubbing instead.
Once you have isolated the process step as the major suspect, it’s time to evaluate it for possible involvement. Look at the following:
Process Chemical Make-up
See if any changes to the process chemistry make-up have been made. Don’t take verbal assurances that “nothing has changed” for gospel. Have an analysis made and look for contaminants that are known to cause adhesion problems (e.g., hexavalent chromium in the cleaner, or high copper concentration in the acid dip, which can produce immersion deposits that don’t adhere well).
Cleaner formulations may change without your knowledge. There is always an effort afoot to reduce the level of chelates present in cleaners (to make waste treatment easier). Reduction of chelate concentrations or substitution of chelates can result in solved waste treatment problems and created adhesion problems.
Heat-treated ferrous parts may yield adhesion problems if the parts produce a large amount of smut as a result of high carbon content at the surface (such as in case hardening). Heat-treated parts may also evidence heat treat scale (oxides) that ordinary cleaning and pickling cannot remove.
For case hardened steels or cast iron, if the scrubbing procedure has pointed to the de-smut step, you will need to look at the chemical make-up and operational parameters of that step.
Acid dips contaminated with metals that are more noble than the basis metal will lead to immersion deposits that yield poor adhesion. Keep a chart of the electromotive series or of a galvanic series in an acidic solution handy. The accompanying table is a typical galvanic series. Note that copper contamination in an acid used to pickle steel can cause copper immersion over the steel parts.
Process Operational Parameters
Many shops also have a program of energy conservation, so the cleaner may be operated at lower than normal temperature.
The cleaner performance is related to chemical make-up (including impurity level), temperature, agitation level and time. Verify that all four of these are within operational specifications and make the appropriate correction(s), one at a time, to resolve the problem.
Problem(s) With Parts
If none of the scrubbing substitutions has resulted in well-adhering deposits, it’s time for a closer look at the parts. Get a history of how the part was produced. Has the manufacturer recently changed manufacturing processes, such as using a new coolant, lubricant or metals suppler? Obtain information as to how the parts were handled for heat treating (if applicable). Have a metallurgical examination made of the basis metal to verify composition, and also determine the structure of the basis metal at and near the surface.
Get as much information as possible on the lubricants used in the manufacture of the parts, and if any formulation changes, such as the addition of boundary additives to lubricants, have been made. Boundary additives chemically bond to steel (remember those ads with automobile engines running with no oil in the crankcase?) and are very difficult to remove.
For heat-treated steel, scrubbing may not effectively remove heavy layers of scale (see photo). You may need to verify that the scale has been removed by the cleaning/pickling cycles ahead of plating by preparing a metallurgical cross section of the suspect parts and looking for heat treat scale with a microscope. A commercial laboratory or a university laboratory (if it has a metallurgy department) can be engaged to help you out, if you do not have this capability.
Frank Altmayer is a Master Surface Finisher, an AESF Fellow, and the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in metal finishing. He received the AESF Past Presidents Award, NAMF Award of Special Recognition, AESF Leadership Award, AESF Fellowship Award, Chicago Branch AESF Geldzahler Service Award, and NASF Award of Special Recognition.

































