In recent years, magnesium (Mg) has evolved as a salient material, in affiliation with electroless nickel (Ni) coating, which have found applications in automobiles, aerospace and confederate fields attributing to its excellent inherent weight sensitive properties.
 Ayushi Thakur and Balasubramanian Kandasubramanian.However, being acknowledged for its remarkable auxiliary properties like flexible machining, appreciable weight sensitivity and ability to be patently die-cast into mesh constructs, magnesium is prejudiced by aeronautical standards predominantly for its inferior corrosion resistance properties. In this sense, electroless nickel plating on magnesium and its alloys has been suggested to extricate it from corrosion problem and make it more competitive in industrial and defence applications. Autocatalytic fixation of metal ions onto respective substrates accrues and alters their mechanical, electrochemical and tribological properties, destitute of any electric current aid. This proficiently identified technique is prosecuted with the assistance of a series of sequenced operations involving a prior pretreatment, which corresponds to the chemical cleaning of the substrate surface; electroless coating; and a later activation process which is a mild etching of the electroless coated surface. The susceptibility of magnesium to this methodology has advanced and propagated its exercise and applicability in aircraft, satellites and allied aeronautical fields.
Ayushi Thakur and Balasubramanian Kandasubramanian.However, being acknowledged for its remarkable auxiliary properties like flexible machining, appreciable weight sensitivity and ability to be patently die-cast into mesh constructs, magnesium is prejudiced by aeronautical standards predominantly for its inferior corrosion resistance properties. In this sense, electroless nickel plating on magnesium and its alloys has been suggested to extricate it from corrosion problem and make it more competitive in industrial and defence applications. Autocatalytic fixation of metal ions onto respective substrates accrues and alters their mechanical, electrochemical and tribological properties, destitute of any electric current aid. This proficiently identified technique is prosecuted with the assistance of a series of sequenced operations involving a prior pretreatment, which corresponds to the chemical cleaning of the substrate surface; electroless coating; and a later activation process which is a mild etching of the electroless coated surface. The susceptibility of magnesium to this methodology has advanced and propagated its exercise and applicability in aircraft, satellites and allied aeronautical fields.
Contemporarily, researchers have proposed various eco-friendly and modified duplex and composite coatings which have transmuted properties of these appendages by tailoring alloy compositions and reagents employed. This review article systematically colligates various considerations and evaluations on electroless nickel applications of magnesium and its alloys and explicates how it anchors its practice in the respective domains. Furthermore, a comprehensive analysis is devised based on the pre-existing treatment methods for accomplishing the same. Image courtesy of Metal Chem.
1. Introduction
Magnesium has evolved and established its importance way back since World War II, where it was employed as an important metal construct for Germany's military aircraft, attributing to its exceptional inherent properties [1,2]. It exhibit competent strength to weight ratio and qualified density, for its increasing demand and applicability in the automobiles, aeronautics, and confederate fields, having a density of 1.74 g⋅cm−3 [3,4], 65% less than aluminium (Al) [5], it replaces metals like ferrous (Fe) and Al, which are stipulated edifice materials for aerospace engineering applications. Being lighter, it unfolds the complications in curtailing fuel consumption and weight reduction of vehicles, which is a great concern in the contemporary world [6,7]. Yet, in defiance of its numerous acknowledged properties, Mg is prejudiced for its vulnerability to environmental corrosion, flammability, poor creep resistance, poor wear resistance and high affinity for reactive solutions [5,[8], [9], [10]].
Contemporarily, poor corrosion resistance is the predominant concern which arises due to its high electronegativity and the oxide/hydroxide layer that negligently protects the concealed metal, circumscribing its applicability to a large extent (E0 = −2.37 V) [8,11]. Moreover, the situation becomes more afflictive for alloys incorporating cerium, lanthanum, aluminium, zinc, yttrium etc. because of reduced homogeneity in the microstructure and phase across the substrate surface, resulting in the development of potential gradient, initiating the consequent corrosive action [[12], [13], [14]]. The corrosion phenomena in Mg, distinctive to those of conventional metals corresponds to Ref. [15] the hydrogenation of Mg on being exploited by the vicinal hydronium ions, resulting in the generation of divalent Mg ions which have high affinity for reactive media (Fig. 1). Moreover, the evolution of hydrogen in acidic media (equation (1)) and alkalization (equation (2)) in neutral or basic media determines the pH on the surface [15].
Mg + 2H+ → Mg2+ + H2 (in acidic solution) (1)
Mg + 2H2O → Mg2+ + 2OH− + H2 (in basic or neutral solution) (2)
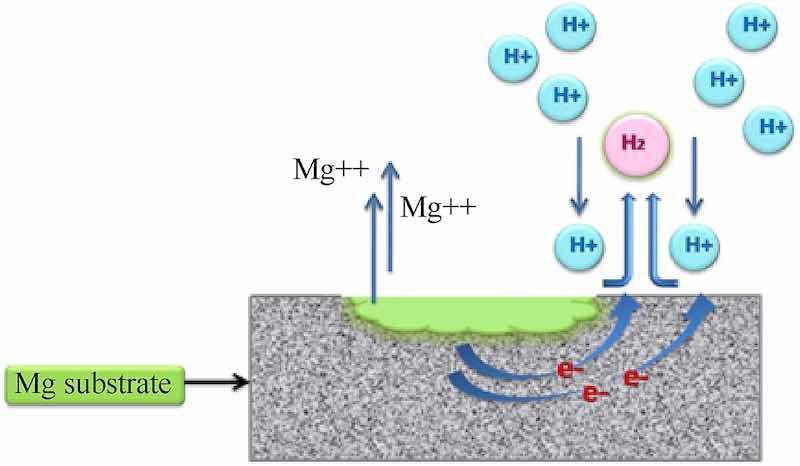 Fig. 1. Corrosion mechanism on magnesium substrate surface (Acidic media).
Fig. 1. Corrosion mechanism on magnesium substrate surface (Acidic media).
Therefore, to obviate these detrimental corrosive actions on magnesium, many protection strategies have been proposed which involve alloying, refining microstructure [8] and most noteworthy, encrusting the substrate with systematized metallic, non-metallic, organic [16] or composite coatings [8,13,17,18].
With the advent of neoteric fabrication techniques, thin metallic [19,20] coatings have enticed much recognition over past decade as they facilitate wider domains attributing to their applicability and economic efficiency. Fixation of metal ions onto any substrate via aqueous solutions can be achieved by electrolytic and electroless techniques [21] as shown in Fig. 2. The latter was formulated by A. Brenner and G. E. Riddell in 1946 [22], for fabricating metal ions onto specific substrates by autocatalytic reduction method, devoid the aid of electric current. In contrast with electroplated ones, electroless deposits have remarkable throwing power and validate uniformity even on the most intricate surfaces [11,23,24] yielding an appreciable thickness and imparting conductance, corrosion resistance, hardness, and other paramount properties to the substrate material, that too without using the expensive electric power assistance. Electroless plating has been demonstrated evidently on a wide range of materials like metals, alloys, polymers, ceramics, composites etc. [[25], [26], [27], [28], [29]], accruing their electrochemical, microstructural and tribological properties. The metals, primarily employed in this methodology are nickel, copper, cobalt, gold, palladium and others selective of the regime of requisite properties [30].
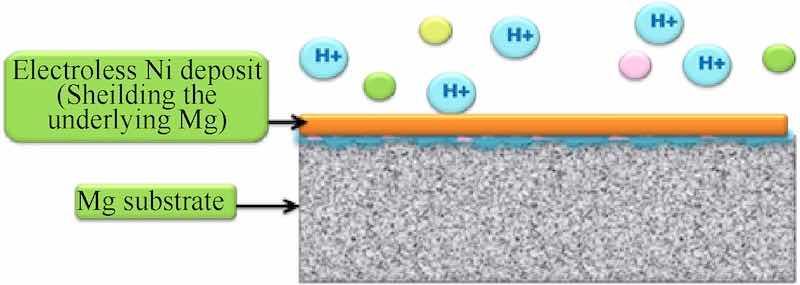
Fig. 2. Electroless deposit on magnesium substrate.
Attributing to the success of this competent methodology, electroless nickel deposition has acquired substantial recognition in academic and industrial domains owing to its excellent coating properties, like fast plating rate [24], uniform deposition, corrosion resistance, solderability, unbeatable surface finish [24], wear characteristics and enhanced tribological properties [31]. Furthermore, this technique has been advantageously practiced on several metal-based alloys, involving steel [[32], [33], [34], [35]], aluminum [36], magnesium etc. [24], in addition to composites [37], ceramics and fibers [[38], [39], [40], [41], [42], [43], [44]]. Contemplating magnesium, one of the salient materials evolved due to its commendable inherent properties, electroless Ni coating has manifested its role in stimulating its applicability and exercise especially as per the aeronautical standards. Hence, electroless Ni deposition methods are extensively utilized, as they impede and barricade the substrate surfaces from pernicious atmosphere extending their life and endurance.
This has also been achieved on alloyed substrates like Mg/Ni hybrid foams [45] and Mg-Li alloys. Mg-Li alloys have been considerably favored as they are known to reduce the aircraft weight to approximately 25%, but they are more vulnerable to environmental corrosion than conventional Mg alloys [46]. Therefore, in assistance with the advanced electroless plating techniques and selective collaboration with heavy metal impurity alloys like Fe, Ni, Cu, the impact of corrosion may be annihilated [47]. The coating properties may be reoriented and refined by customizing alloy compositions and since the electrochemical potential of Ni is considerably higher than that of Mg, this combination fits perfectly under the category of cathodic plating on the anodic substrate [48]. Integrating other worthy elements into nickel plating such as Phosphorus [48,49], Boron [50], Copper [51], Carbon [52], Cobalt [53] etc., electroless co-deposits have proved to enhance their caliber and applicability in association with Mg substrates [24]. Predominantly Ni-P [48,54] binary alloy deposits have gained consideration over other coatings attributing to their superior corrosion resistive properties. Besides, Ni-B [50] binary coatings and developed duplex and ternary coatings like Ni-P-B, Ni-Co-P, Ni-Cu-P have also been formulated and attentively considered for the Mg substrates to have superior corrosion resistant, microstructural and tribological properties. However, sometimes in order to bestow superior structural properties to the substrates, in addition to the primary electroless Ni film, a Micro Arc Oxidation (MAO) interlayer is introduced and sandwiched between the substrate and the coating surfaces [[43], [44], [45], [46], [47]]. The following flowchart (Fig. 3) summarizes the variety of electroless nickel coatings, beneficially experimented on magnesium substrates [24,55].
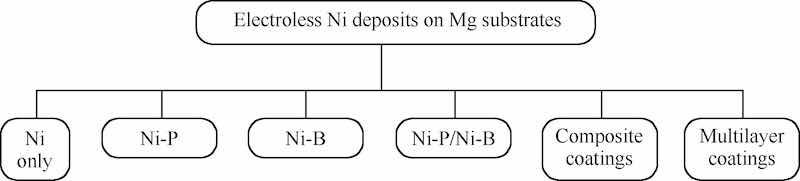
Fig. 3. Electroless nickel coatings on magnesium substrates.
Therefore, in affiliation with electroless metal deposition technique, the surfaces of Mg substrates can be profoundly fabricated and engineered to augment their usability and exercise by many folds [8]. This review aims at highlighting the fundamental aspects of electroless Ni deposition on various Mg substrates and their impact in extensively reinforcing their anticorrosive and tribological properties. The innovative formulations and strategies from various researchers have been cataloged describing their role in making Mg components outlast in the most stringent environmental conditions in airborne and space applications. Alongside, the specifications and implementations of the technique have been briefly enumerated, the pre-existing abstruse procedures have been described in the review.
2. Technical standards and specifications
Since electroless deposits physically barricade the substrate surface from the attacking environment, they have to be uniform, intact and fairly adhesive which makes them more convenient and favorable than electroplated deposits. Chemically reduced Ni ions are deposited onto the catalytic Mg substrate surface after a series of pretreatment steps and the necessity of these arises due to high affinity of Mg to form oxide/hydroxide layers, sensitivity towards belligerent attack of electrolytic components of the soaking medium, in addition to the low surface chemical homogeneity because of the presence of alloying elements thereby creating a phase difference and consequently, a potential gradient across the substrate surface [12,56].
Hence, before immersing the substrate an electroless bath, it is exposed to a series of pretreatment steps, exfoliating the substrate surface which requires to be cleansed of all impurities including dirt, grease and subsisting oxide/hydroxide layers, which may obstructively interfere with the coating process, creating problems in adhesion, compaction and uniformity of the coating, causing prior failure of the coating and subsequently the concealed metal. The generic pretreatment process of electroless Ni deposition is illustrated in Fig. 4 [13,56,57], and the electroless Ni bath formulations have been extensively revised expediently over the years by the configurational identified reagents.
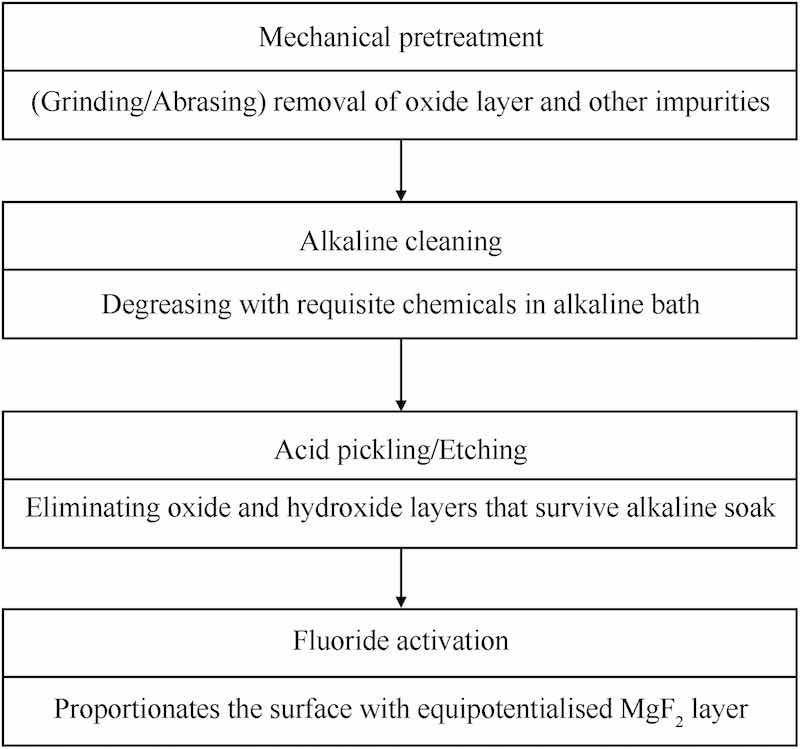
Fig. 4. Schematic depiction of the electroless pretreatment process.
2.1. Pretreatment processes
Mg substrate surface is furnished with coveted properties prior to electroless nickel deposition, which can be achieved by mechanical brushing, abrading, grinding or polishing followed by chemical cleaning and pickling, primarily employed for scraping off the oxide/hydroxide grease, or any other despicable impurity [58].
Mechanical pretreatment: This is chiefly undertaken for removing oxide/hydroxide, dirt or unwanted deposits by simply polishing, grinding, brushing or abrading in order to obtain the desired surface finish on the candidate surface before exposing it to the chemical treatments. SiC emery papers of grit size 1000, 2000, 5000 or Al2O3 of mesh size 60–400 [56] are employed for various investigations in order to scrap off the undesired layer.
Sensitization: Subsequent to the prevenient mechanical coverage the substrate is exposed to alkaline sink wherein it is extricated of the greasy, oily and organic impurities, encouraged by the alkalis like NaOH, Na3PO4 etc. [25]
Pickling/Acid etching: The oxides/hydroxides which survive the alkaline cleaning treatment are eliminated by the virtue of acid etching which entails either acidic or neutral pH conditions. This step helps in increasing surface roughness within certain limits which is important for smooth, uniform and compact adhesion of the coating, in addition, to increase in plating rate, causing better interlocking between the two films [56].
Activation: An important step for the substrates having heterogeneity in surface phase. Fluoride activation fixates a MgF2 equipotentialised temporary layer which serves as the base for further coatings [56]. It is an important step, although the Hydrofluoric acid employed for the motive has a tendency to root professional hazards. However, A.A. Zuleta et al. [54] activated a commercial purity Mg sample using NH4HF2 which equally toxic to HF but can be somehow comparatively controlled.
2.2. Undercoating
The undercoating is an obligatory attempt to aid smooth and uniform fabrication of the subsequent electroless coating [56] which can primarily be achieved via two routes; zinc immersion, which may be followed up by either electroplating or electroless plating, and secondly direct Ni electroless plating which may be a prior step to an electroplating deposition [59]. Sometimes, Changdong Gu et al. [3] for the very first time experimented direct electroless Ni plating after fluoride activation on AZ91D Mg alloy and studied the nucleation mechanisms on Ni-P deposits. On the other hand zinc immersion, although not specifically recommended for magnesium alloys having high aluminum content, is a mutual operation for electroless as well as electroplating [56] and is condemned as it needs to be controlled precisely. In assistance with ultrasonic agitation, the zincating treatment can also multiply the nucleation sites which refines the coating layer adhesion [60,61].
Sometimes conversion layers may also be employed for undercoating purpose which later dissolves as immersed in electroless media paving way for Ni ions to fixate. A Mg2P2O7 pretreatment layer was formulated by Shao et al. on AZ91D Mg substrate, which resulted in a consistent and smooth electroless Ni deposition subsequently [62].
Several challenges may be encountered by the subsequently deposited layer, in case if the undercoating has high porosity and non-uniformity, which is more exigent when magnesium substrates are concerned. Copper immersion as another process which could not prosper mainly because of its inconsistency in thickness as it was electrodeposited [13,56] thus, operating a cyanide bath, a dormant Cu layer can be deposited on zinc coating to facilitate successive electroless plating on the substrate.
2.3. Electroless nickel bath characteristics
An electroless nickel bath comprises a certain set of chemicals, each having a specific function to perform as tabulated in Table 1 [24,50,55,63]. Moreover the controlled compositions of these chemicals regulate the fundamental bath characteristics and ultimately the comprehensive properties of the electroless coats.
Table 1. The constituent chemicals comprising an electroless nickel bath and their functionality.
| Component | Chemical | Performance |
| Nickel ions | Nickel hypophosphite | Source of the metal deposit |
| Reducing source | Electron source | |
| Buffers | Sodium carbonate and sodium phosphate [64] | pH regulation |
| Stabilizer | Succinic acid [54] | Stabilizes the solution |
| Surfactants | Ammonium Lauryl Sulfate [65] | Lower surface tension; increase wettability |
| Complexing Agent | Tetrasodium pyrophosphate and ammonium citrate [66] Ethylenediamine [55] | Prevent precipitation of Ni salts; Form complexes with metals |
| Accelerators/Exultant | Reducing agent activation |
2.4. Selectivity and preference of reducing agent
Reducing agents are the fundamental sources of electrons warranted by the standard specifications of the electroless baths, to carry out the reduction mechanism by getting oxidized themselves. Miscellaneous reducing agents have been employed for assembling an electroless nickel bath depending upon the type and demand of coating required, some of which, syndicated with magnesium alloys have been enlisted in Table 2 [24,55]. Sodium hypophosphite is the most coveted and illustrious reducing chemical especially on a commercial scale, ascribing to its economic efficiency, exceptional corrosion resistance, and uncomplicated bath management [24,55]. On the other hand, hydrazine reduced coatings show very less industrial applicability due to high expenses and complexity in bath controlling [55].
Table 2. Reducing agents for corresponding deposits.
| Reducing agent | Ni coating |
| Hydrazine (NH 2 NH2) | Ni |
| Sodium Hypophosphite (NaH2PO2) | Ni-P |
| Sodium Borohydride (NaBH4) | Ni-B |
| Aminoboranes | Ni-B |
| DMAB (Dimethylamine borane) | Ni-B |
3. Nickel phosphorus deposits
The primeval hypophosphite reduced electroless nickel deposits have been extensively researched and commercialized over the years on account of their exceptional anti-corrosive and tribological properties in addition to favorable adhesion, hardness, solderability, plating rate, and thickness consistency. Availability of wide-ranging literature and study on Ni-P binary alloy deposits in association with magnesium alloys explains their scope and effectuality as per industrial, automotive and aerospace standards.
The preparation and formulation methods may involve either acidic or alkaline baths [55] corresponding to 5 wt%-14 wt% P and 1 wt%-4wt% P content, respectively [56]. Evidently, the content of phosphorus in the deposit determines the structure and consequently other aforementioned properties of the coating, thereby governing its strength and performance. The EDX and SEM magnifications have validated crystalline and amorphous microstructures for low and high P contents respectively, and also amorphous and crystalline for mid P content samples. The morphological evaluations of the amorphous Ni-P deposit on Mg substrate surfaces yield a nodular microstructure resembling a cauliflower, which offers superior hardness corrosion resistance and mechanical properties in comparing with crystalline forms [59,67,68].
It has also been noted that the coating rate is directly proportional to the temperature until a certain threshold is surpassed [66]. Moreover, the most compatible and beneficial Ni-P coatings are achieved usually at low pH values around 6.5 [69] and indeed once the sample is sensitized and prepared, the electroless films should be immediately deposited so as to avoid the formation of oxide/hydroxide layer on the Mg substrate which may cause problems in adhesion [54].
3.1. Corrosion resistance
Rather than offering a sacrificial action, electroless Ni deposits barricade the Mg substrate surface from the external environment and protect them from consequent corrosion. The corrosion protecting potentials of these deposits can be measured by salt spray or immersion tests most commonly using 3.5 wt% and 5 wt% NaCl solution [49,68,70] and artificial sweat solutions (pH4.8-5.8) [71] and thereafter evaluating the potentiodynamic curves obtained.
W.J. Cheong et al. [72] reported the dependence of corrosive action on morphological aspects rather than microstructural properties, by employing Maleic acid (MA) and Thiourea (TU) stabilizers in the electroless system. The thermal treatments which are undertaken in order to accrue the hardness values of the deposits sometimes evince to be detrimental in terms of corrosion protection due to the microcrack formation during the process [73].
3.2. Mechanical and tribological properties
Manifold and miscellaneous conveniences of hypophosphite which reduced Ni deposits on magnesium substrates have made them more extensive and widespread, in fact in order to achieve a higher level of perfections, these have also been modified into composite and multilayer systems which will be comprehended in later sections. The coating layer thickness which increases with the application time [74] must be consistent and compact with tensile strength more than 12 MPa, for favorable and fine adhesion [61]. Thermal treatments are suggested to enhance the hardness and mechanical performances of the Ni-P coatings along with the addition of hard particles like Al2O3, diamond and SiC [65] and smooth uniform microstructure at 6.5 pH [69]. Improved hardness values 660 VHN [3], 763 HV [48], 915 HV [67] have been recorded along with the appreciable coefficient of friction as low as 0.14 [69] for varied magnesium alloys.
4. Nickel boron deposits
Although a large number of literature is available on hypophosphite reduced Ni-P coating systems, Scientists have also developed borohydride and aminoborane reduced Ni-B coatings which resemble the former except for some few exceptional distinctive properties. Ni-B deposits, being more costly than Ni-P ones have enticed some attention in the past decade due to their superior abrasion and wear resistance properties in addition to good brazing, solderability, and ultrasonic bonding attributes.
The formulations and specifications of acidic or alkaline electroless bath determine the boron content in the coating, varying 0.1%–4% in acidic DMAB or DEAB bath [55], 0.2%–4% in aminoborane, 4%–7% in sodium borohydride [55], and exposure to thermal treatment yields precipitated Ni, NI3B, Ni2B, and Ni4B3 [64] compounds as a result of amorphous to crystalline phase transition. Simultaneously undergoing reactions release hydrogen and the following reaction [64] has been recorded during high Ni ion concentration condition in the electroless bath (equation (3)).
4Ni2+ + 8BH−4 +18H2O → 2Ni2B + 6H3BO3 + 25H2 ↑ (high Ni ion conc.) (3)
Although they exhibit a lower level of corrosion resistance as compared to Ni-P deposits, they are well suited and recommended for their high hardness, abrasion resistance, and anti-wear performances. These nickel-boron deposits have been intrinsically investigated on substrates like glass, plastics, steels, aluminum, and so on, to comprehend their tribological and hardness performances and indeed have witnessed a hardness as high as 1000hk100 Al on knoop scale [50]. For magnesium since corrosion is the predominant concern, very few standardized applications necessitate Ni-B coatings, hence these are either directly being plated or duplexed with Ni-P films.
Correa et al. [75] witnessed increased anti-wear and hardness performances of Ni-B coatings on pure commercial Mg and AZ91D Alloys procuring a consistent coating thickness ranging between 13-16 μm and 12–15 μm respectively, with boron content approximating 5 wt%. The hardness value of Ni-B plated AZ91D Mg alloy was 616 HK0.05, considerably more than 563 HK0.05, obtained on commercial purity Mg. Optimum concentrations of sodium borohydride a nickel acetate have begotten more firm, compact and uniform deposited films with faster rates, although unmatched with Ni-P deposits; Also the measured increase in boron content has stimulated enhancement in hardness performance unlike in the case of Ni-P coatings [76].
5. Nickel-phosphorus/Nickel-boron duplex deposits
Individually fabricated electroless protective layers on magnesium and other substrates are widely identified and accepted accrediting to their beneficial characteristics. Nevertheless, scientists have innovatively approached and engineered duplex layering systems on such substrates and selectively utilized the qualitative attributes of these in order to procure amplifying results. One of such approaches has been successively embraced by assembling Ni-P/Ni-B duplex deposits on magnesium substrates which have yielded appreciable results in terms of corrosion and tribological properties [77].
Ni-P/Ni-B duplex coatings are deliberately performed on magnesium alloys for integrating adequate corrosion resistance with appreciable wear resistance. Generally, the duplex systems are prepared by employing dual electroless baths comprising acidic hypophosphite and alkaline borohydride respectively. Ni-P and Ni-B layer can be allocated either interiorly or posteriorly depending upon the requisite attributes. The coating layer exposed to the environment dominates its principle trait over the other as illustrated in Fig. 5. W.X Zhang et al. [77] fabricated a duplex Ni-P-B layer on AZ91D Mg alloy substrate, having interior Ni-P film 20 μm and the exterior Ni-B film 15 μm thick, and confirmed the improved anti-corrosive and hardness utilities when compared with the base substrate and individual coatings.
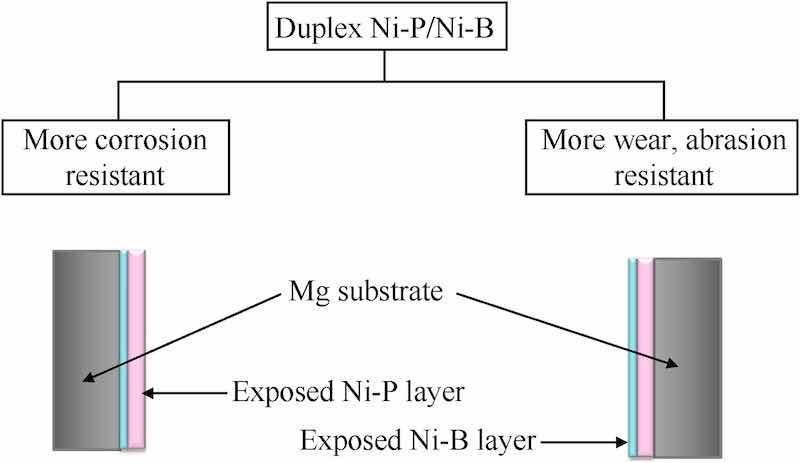
Fig. 5. Preferential duplex Ni-P/Ni-B system.
Furthermore, these duplex systems have also been transformed into complex coatings by the virtue of incorporating metals like Zinc, Copper, Molybdenum etc. [78] and have been commissioned for various naval and hydrodynamic applications [79].
6. Advanced electroless Ni deposits
6.1. Composite coatings
Composite based coatings incorporations in the electroless deposits have been developed and are advanced, preferred attributing to their elevated performance and standards via integrated inter metallic entities into the electroless Ni-P matrix. Scientists have demonstrated this objective by devising ternary coatings such as Ni-W-P [80], Ni-Cu-P [81], Ni-Co-P along with quaternary Ni-W-Cu-P [82] ones and moreover intermittently emphasizing on wear resistance, materials like SiC, Al2O3, WC, B4C or even diamond, PTFE (polytetrafluoroethylene) [24,55], nylon lycra textiles [83] have also been considered as incorporate. However, due to the strenuous deposition mechanism on magnesium alloys, these composite coatings haven't been extensively explored on Mg substrates and are therefore being researched and proffered. The ternary and poly alloy coatings on Mg have enhanced their hardness, wear resistance and anti-corrosion properties to outstanding folds thereby making them more convenient and practical than the basic nickel or binary alloy electroless coatings.
Transition metal tungsten has been suggested as a third element for Ni-P deposits on account of convenient and imperative properties such as superior hardness, frictional behavior, favorable wear and corrosion resistance in addition to its highest melting point imparting beneficial thermal stability [81,84]. Comprehensively, Ni-W-P deposits have been achieved by appending sodium tungstate or tungsten as the source of metal ions into the electroless bath, albeit Shibli et al. [85] used electroless Ni-P plated tungsten powder as the source of tungsten in the coating for mild steel. Considering Mg substrates, the restricted availability of literature confirms how much it needs to discourse. Zhang et al. [80] deposited electroless Ni-W-P on AZ91D Mg substrate, reaffirmed an increase in hardness values and a significant drop in corrosion current density from 411.8 μA⋅cm−2 to 7.809 μA⋅cm−2 along with a dense non-porous nodular structure. Alongside icorr value approximating 0.55 μA⋅cm−2 was also reported by V. Ezhil Selvi et al. [86] for a passivated and heat treated AZ31B Mg alloy sample by duplexing Ni-P with composite Ni-W-P electroless deposit. Double layering of Ni-P with Ni-P-ZrO2 [87], Ni-Zn-Cu [88], Ni-Sn-P [89] or gradient SiC deposits [90], along with introducing Carbon into Ni coatings [52] or Ni-P-C nanotube coatings [91] or nano-composite Ni-P-ZnO [92], Ni-P-TiO2 [93] coatings, are some of the being investigated methodologies which have till date proven to have improved the attributes of traditional accretions with respect to Mg substrates.
Moreover, Copper [94] and Cobalt [53] composites have also been experimented on Mg substrates which shows improved and enhanced characteristics, especially in nickel/metal hydride batteries.
6.2. Sub-structuring interlayer
Sandwiching Interlayers in between the substrate and electroless systems has been progressively researched and performed accounting to their excellent insulating properties. Recent progress in organic interlayers [59], Anodized [58] and Micro Arc Oxidation films [95] are being developed and their fabrication has promised better results as per applicable standards.
Anodizing is an oxidizing mechanism which according to Salman et al. [58] was patented by Bengough in 1923, which facilitates electrolytically transforming a metallic surface into a mask of advantageous characteristics. Shuo Sun et al. [96] ameliorated the corrosive properties of AZ91D Mg alloy of electroless Ni plating along with catalytic TiB2 powder on the pre-anodized substrate surface by employing Dow's 17 technique. Also, the adhesion, wear characteristics, frictional and other requisite improvements in respective properties have been yielded by employing various anodizing techniques familiarized as HAE process, named after the innovator Harry A. Evangelides [58], Tagnite [97], Anomag processes [98] and various other.
On the other hand, Micro Arc Oxidation (MAO) also known as Plasma Electrolytic Oxidation (PEO) is an altered version of anodizing which layers the Mg substrates with oxide films, engaging elevated potentials comparatively. SEM images have manifested the unvaried porous morphology due to agglomerated particulate oxides and inconsistency in the layer thickness which can be selectively controlled by electrolyte composition, power voltage and PH regulation [95]. The process demonstrates initial dissolution of the metal substrate and then eventually increase in deposited mass with time, subsequently final termination into a more uniform fabricated finish [99]. The structural components like MgO, MgAl2O3, MgSiO3 [100], MgSiO4 [101] have been reported. V. Ezhilselvi et al. [102] constructed double MAO/Ni-P and MAO/Ni-P/Ni-W-P deposits on AZ31B Mg alloy and noted the remarkable drop in corrosion current density because of the MAO layer with increased corrosion resistance. However, MAO layer has also been reported to have been affected by the corrosive action of the subsequent electroless layer [103].
7. Applications
Ever since the successful demonstration of Mg components in the military combating aircraft during World War II, magnesium has enticed much attention and acknowledgment persistently through the epoch. This universally recognized metal, for its light weight and authentic strength to density ratio, has established itself as the most recommended metal in the field of automotive, electronics, and aerospace. However, eventually, it was overtaken by other metals like Aluminum and steels on account of its frailties, chiefly the vulnerability to corrosion; until the innovative fabrication methods were formulated which not only reinforced its properties but also broadened the spectrum of their applicability. Imparting good throwing power, hardness, leveled plating thickness, improved solderability along with wear and corrosion resistance, Electro-less Ni plating technique on Mg substrates has advanced its applicability and exercise in the automobile, electronics, aerospace, and confederate fields.
Mg was first employed in automobile industries in 1919 in Germany [58] and ever since has been extensively exercised on account of curtailment in vehicle weight and the concomitant lowering in fuel consumption and Carbon Dioxide emission [13]. In alliance with modern electroless Ni coating technique, Mg automobile industries have recruited Mg for critical assembling components such as steering, interior door frames, seat cases, hind door panels, fuel storages etc. [58]
Mg components remain handy, lightweight and decent sized as per the standards of electronics industries, therefore their demand in the market stays purposefully beneficial [58]. Many manufacturers of leading brands use Mg components in their products such as cameras, laptops, mobile phone handsets, audio players etc. [56,58]. Furthermore, Mg-based hydrogen storage alloys, Ni/metal hydride batteries [53] have also been formulated and fabricated with the assistance of electroless nickel deposits which has optimized and enhanced their performance.
Concerning aerospace applications, Sharma et al. [11] authenticated thermal stability, solderability, thermal cycling and optical properties of electroless nickel plated magnesium substrate, attributing to satellite operations and obtained satisfactory characteristics. No change in optical properties ranging from 0.37 to 0.38 (solar absorbtance) and 0.10–0.12 (infrared emittance) were seen, demonstrating the perfect environmental stability in space also. Electro-less Ni plating being inorganic exhibits very less outgoing, hence the percent values of (total mass loss) TML and CVCM (collected volatile condensable material) were observed to be 0.05% and 0.01% which were much lower than the discrete limits.
Therefore, fabricated Mg serves as an important structural constituent for standard air vehicles and spacecraft involving parts such as gearboxes, oil pumps, fuel tanks, wheels, doorways, seats, antenna for radars, and skins constructs on wings and various other parts. Moreover, magnesium based collector, base plate, and radiator of TWT (traveling wave tube) of the communication satellite, fabricated with electroless Ni-P have been captured by Sharma et al. [11] at ISRO center.
Forbye, Ni/Au deposits on magnesium substrates for aerospace applications have also been witnessed recently [56], and Ni electroless deposits have also been serviceable on engine cranksets, fuel pump cover and shell components in aeronautical applications [61].
8. Conclusion
In the present review, we reported research advancements for improving the performance of magnesium, an advocated metal, by the virtue of fixating thin shielding electroless nickel films on its surfaces. The compositional standards and variations of the electroless and the controlling systems and their influence on the deposit characteristics have been overviewed. From the past decade, refining and evolution of electroless Ni coating systems on magnesium substrates has been a trend of interest for researchers and manufacturers, chiefly on account of its inherent weight sensitive properties. Hence, continuous developments in this strategy have led to the rapid propagation of Mg and its alloys in the electronics, automobile, avionics, and aeronautical domains, making it the most suited and competent candidate in the market.
Written by Ayushi Thakura, Swaroop Ghardeb, and Balasubramanian Kandasubramanianb.
a Department of Metallurgical and Materials Engineering, National Institute of Technology, Hazratbal, Srinagar, Jammu & Kashmir, 190006, India; b Department of Metallurgical & Materials Engineering, DIAT (DU), Ministry of Defence, Girinagar, Pune, 411025, Maharashtra, India
Acknowledgments: The authors would like to thank Dr. C. P. Ramanarayanan, Vice-Chancellor, DIAT, Pune for their constant encouragement and support. Mr. Ramdayal Yadav and Mr. Prakash Gore are also acknowledged for technical assistance and support.
References
[1] Ball CJP. The history of magnesium. J Am Soc Nav Eng 2009;69:81e94. https://doi.org/10.1111/j.1559-3584.1957.tb04052.x.
[2] Mathaudhu SN, Luo AA, Nyberg EA, Neelameggham NR, Silleken WH. Essential readings in magnesium technology. Cham: Springer International Publishing; 2016. https://doi.org/10.1007/978-3-319-48099-2.
[3] Gu C, Lian J, Li G, Niu L, Jiang Z. Electroless Ni - P plating on the AZ91D magnesium alloy. J Chin Soc Corros Prot 2005;25:271e4. https://doi.org/ 10.1016/j.jallcom.2004.07.083.
[4] Gu C, Lian J, He J, Jiang Z, Jiang Q. High corrosion-resistance nanocrystalline Ni coating on AZ91D magnesium alloy. Surf Coating Technol 2006;200: 5413e8. https://doi.org/10.1016/j.surfcoat.2005.07.001.
[5] Hombergsmeier E. Magnesium for aerospace applications. In: Second Int Conf Exhib“‘Magnesium Broad Horizons’”; 2007. p. 1e13.
[6] Gusieva K, Davies CHJ, Scully JR, Birbilis N. Corrosion of magnesium alloys: the role of alloying. Int Mater Rev 2015;60:169e94. https://doi.org/10.1179/ 1743280414Y.0000000046.
[7] Lun Sin S, Elsayed A, Ravindran C. Inclusions in magnesium and its alloys: a review. Int Mater Rev 2013;58:419e36. https://doi.org/10.1179/ 1743280413Y.0000000017.
[8] Esmaily M, Svensson JE, Fajardo S, Birbilis N, Frankel GS, Virtanen S, et al. Fundamentals and advances in magnesium alloy corrosion. Prog Mater Sci 2017;89:92e193. https://doi.org/10.1016/j.pmatsci.2017.04.011.
[9] Guo KW. A review of magnesium/magnesium alloys corrosion and its pro- tection. Recent Pat Corros Sci 2010;2:13e21. https://doi.org/10.2174/ 1877610801002010013.
[10] Mordike B, Ebert T. Magnesium: properties-applications-potential. Mater Sci Eng, A 2001;302:37e45. https://doi.org/10.1016/S0921-5093(00)01351-4.
[11] Sharma AK, Suresh MR, Bhojraj H, Narayanamurthy H, Sahu RP. Electroless nickel plating on magnesium alloy. Met Finish 1998;96:10e9.
[12] Liu Z, Gao W. Electroless nickel plating on AZ91 Mg alloy substrate. Surf Coating Technol 2006;200:5087e93. https://doi.org/10.1016/ j.surfcoat.2005.05.023.
[13] Gray JE, Luan B. Protective coatings on magnesium and its alloysd a critical review. J Alloy Comp 2002;336:88e113. https://doi.org/10.1016/S0925- 8388(01)01899-0.
[14] Meng J, Sun W, Tian Z, Qiu X, Zhang D. Corrosion performance of magnesium (Mg) alloys containing rare-earth (RE) elements. In: Corros. Prev. Magnes. Alloy. Elsevier; 2013. p. 38e60. https://doi.org/10.1533/ 9780857098962.1.38.
[15] Song G-L. Corrosion behavior and prevention strategies for magnesium (Mg) alloys. In: Corros. Prev. Magnes. Alloy. Elsevier; 2013. p. 3e37. https:// doi.org/10.1533/9780857098962.1.3.
[16] Bai K, Zhang Y, Fu Z, Zhang C, Cui X, Meng E, et al. Fabrication of chitosan/ magnesium phosphate composite coating and the in vitro degradation properties of coated magnesium alloy. Mater Lett 2012;73:59e61. https:// doi.org/10.1016/j.matlet.2011.12.102.
[17] Hu R-G, Zhang S, Bu J-F, Lin C-J, Song G-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog Org Coating 2012;73:129e41. https://doi.org/10.1016/j.porgcoat.2011.10.011.
[18] Ren M, Cai S, Liu T, Huang K, Wang X, Zhao H, et al. Calcium phosphate glass/ MgF2 double layered composite coating for improving the corrosion resis- tance of magnesium alloy. J Alloy Comp 2014;591:34e40. https://doi.org/ 10.1016/j.jallcom.2013.12.215.
[19] Faraji S, Faraji AH, Noori SR. An investigation on electroless Cu-P composite coatings with micro and nano-SiC particles. Mater Des 2014;54:570e5. https://doi.org/10.1016/j.matdes.2013.08.092.
[20] Faraji S, Rahim AA, Mohamed N, Sipaut CS, Raja B. The in uence of SiC particles on the corrosion resistance of electroless, Cu-P composite coating in 1 M HCl. Mater Chem Phys 2011;129:1063e70. https://doi.org/10.1016/ j.matchemphys.2011.05.060.
[21] Walsh FC, Ponce de Leon C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Trans IMF 2014;92:83e98. https://doi.org/ 10.1179/0020296713Z.000000000161.
[22] Sawyer C. The pH-responses of electrodes prepared from glasses of widely different compositions have been compared with the hygroscopicity of the glasses (Technical News. Publ Natl Bur Stand 1947:75.
[23] Kerr C, Barker D, Walsh F. Electroless deposition of metals. Trans IMF 2001;79:41e6. https://doi.org/10.1080/00202967.2001.11871359.
[24] Sahoo P, Das SK. Tribology of electroless nickel coatings - a review. Mater Des 2011;32:1760e75. https://doi.org/10.1016/j.matdes.2010.11.013.
[25] Gharde S, Kandasubramanian B. The importance of electroless metallic build- up on surface modi ed substrates for multifunctional engineering applica- tions: a recent progress update. Trans Indian Inst Met 2018;71:2873e92. https://doi.org/10.1007/s12666-018-1397-6.
[26] Yadav R, Balasubramanian K. Metallization of electrospun PAN nano bers via electroless gold plating. RSC Adv 2015;5:24990e6. https://doi.org/10.1039/ c5ra03531g.
[27] Khanale M, Balasubramanian K. Molecular simulation of geometrically optimized polyoxymethylene/poly (vinylalcohol) gel membrane for elec- troless scrubbing Ni(II) ions. J Environ Chem Eng 2016;4:434e9. https:// doi.org/10.1016/j.jece.2015.11.044.
[28] Arora R, Singh N, Balasubramanian K, Alegaonkar P. Electroless nickel coated nano-clay for electrolytic removal of Hg(ii) ions. RSC Adv 2014;4:50614e23. https://doi.org/10.1039/C4RA06988A.
[29] Kandasubramanian B. EMI properties of electroless nickel-coated mica in different polymer matrices. PhD thesis. Loughborough University; 2003.
[30] Henry JR. Electroless (autocatalytic) plating. Met Finish 2002;100:409e20. https://doi.org/10.1016/S0026-0576(02)82044-X.
[31] Shoeib MA, Mokhtar SM, Abd El-Ghaffar MA. Mechanical and corrosion protection properties of electroless nickel-polymer composite coatings. Met Finish 1998;96:58e9. https://doi.org/10.1016/S0026-0576(98)80873-8.
[32] Liu R, Gao C, Yang J. High corrosion-resistant and long-life-span electroless nickel process. Met Finish 2002;100:34e7. https://doi.org/10.1016/S0026- 0576(02)80296-3.
[33] Shibli SMA, Remya R, Beevi AA. Electrochemical characterisation of process of ruthenium based surface activation for electroless nickel plating. Trans IMF 2007;85:147e52. https://doi.org/10.1179/174591907X192285.
[34] Shibli SMA, Jabeera B, Anupama RI. Incorporation of nano zinc oxide for improvement of electroless nickel plating. Appl Surf Sci 2006;253:1644e8. https://doi.org/10.1016/j.apsusc.2006.02.063.
[35] Shibli SMA, Dilimon VS. Effect of phosphorous content and TiO2TiO2- reinforcement on NieP electroless plates for hydrogen evolution reaction. Int J Hydrogen Energy 2007;32:1694e700. https://doi.org/10.1016/ j.ijhydene.2006.11.037.
[36] Court S, Kerr C, Ponce de Leon C, Smith JR, Barker BD, Walsh FC. Monitoring of zincate pre-treatment of aluminium prior to electroless nickel plating. Trans IMF 2017;95:97e105. https://doi.org/10.1080/ 00202967.2016.1236573.
[37] Zhang Q, Wu M, Zhao W. Electroless nickel plating on hollow glass micro- spheres. Surf Coating Technol 2005;192:213e9. https://doi.org/10.1016/ j.surfcoat.2004.06.013.
[38] Gore PM, Kandasubramanian B. Functionalized aramid bers and composites for protective applications: a review. Ind Eng Chem Res 2018;57:16537e63. https://doi.org/10.1021/acs.iecr.8b04903.
[39] Deoray N, Kandasubramanian B. Review on three-dimensionally emulated ber-embedded lactic acid polymer composites: opportunities in engineer- ing sector. Polym Plast Technol Eng 2018;57:860e74. https://doi.org/ 10.1080/03602559.2017.1354226.
[40] Gore PM, Kandasubramanian B. Heterogeneous wettable cotton based superhydrophobic Janus biofabric engineered with PLA/functionalized- organoclay micro bers for ef cient oilewater separation. J Mater Chem 2018;6:7457e79. https://doi.org/10.1039/C7TA11260B.
[41] Gore PM, Khurana L, Siddique S, Panicker A, Kandasubramanian B. Ion- imprinted electrospun nano bers of chitosan/1-butyl-3-methylimidazolium tetra uoroborate for the dynamic expulsion of thorium (IV) ions from mimicked ef uents. Environ Sci Pollut Res 2018;25:3320e34. https:// doi.org/10.1007/s11356-017-0618-6.
[42] Sharma S, Balasubramanian K. Molecularly imprinted and nanoengineered camphor soot functionalized PAN-nano bers for ef uent treatment. RSC Adv A. Thakur et al. / Defence Technology 15 (2019) 636e644 643 2015;5:31732e41. https://doi.org/10.1039/C5RA02861B.
[43] Balasubramanian K, Yadav R, Prajith P. Antibacterial nano bers of polyoxy- methylene/gold for pro-hygiene applications. Int J Plast Technol 2015;19: 363e7. https://doi.org/10.1007/s12588-015-9127-y.
[44] Badhe Y, Balasubramanian K. Nanoencapsulated core and shell electrospun bers of resorcinol formaldehyde. Ind Eng Chem Res 2015;54:7614e22. https://doi.org/10.1021/acs.iecr.5b00929.
[45] Liu J, Shi S, Zheng Z, Huang K, Yan Y. Characterization and compressive properties of Ni/Mg hybrid foams. Mater Sci Eng, A 2017;708:329e35. https://doi.org/10.1016/j.msea.2017.09.130.
[46] Sun Y hua, Wang R chu, Peng C qun, Feng Y, Yang M. Corrosion behavior and surface treatment of superlight MgeLi alloys. Trans Nonferrous Met Soc China (English Ed 2017;27:1455e75. https://doi.org/10.1016/S1003- 6326(17)60167-5.
[47] Salvago G, Fumagalli G, Brunella F. Corrosion behaviour of electroless Ni-P coatings in chloride-containing environments. Surf Coating Technol 1989;37:449e60. https://doi.org/10.1016/0257-8972(89)90083-2.
[48] Seifzadeh D, Mohsenabadi HK, Rajabalizadeh Z. Electroless Ni-P plating on magnesium alloy by innovative, simple and non-toxic oxalate pretreatment and its corrosion protection. RSC Adv 2016;6:97241e52. https://doi.org/ 10.1039/c6ra19984d.
[49] Rajabalizadeh Z, Seifzadeh D. Application of electroless Ni-P coating on magnesium alloy via CrO3/HF free titanate pretreatment. Appl Surf Sci 2017;422:696e709. https://doi.org/10.1016/j.apsusc.2017.06.100.
[50] Delaunois F, Petitjean JP, Lienard P, Jacob-Duliere M. Autocatalytic electroless nickel-boron plating on light alloys. Surf Coating Technol 2000;124:201e9. https://doi.org/10.1016/S0257-8972(99)00621-0.
[51] Wu L, Zhao J, Xie Y, Yang Z. Progress of electroplating and electroless plating on magnesium alloy. Trans Nonferrous Met Soc China 2010;20:s630e7. https://doi.org/10.1016/S1003-6326(10)60552-3.
[52] Mao Y, Li Z, Feng K, Guo X, Zhou Z, Dong J, et al. Preparation, characterization and wear behavior of carbon coated magnesium alloy with electroless plating nickel interlayer. Appl Surf Sci 2015;327:100e6. https://doi.org/ 10.1016/j.apsusc.2014.11.151.
[53] Li Y, Wang C, Dong Z, Wang J, Yang S, Ke D, et al. Effects of coating layers on electrochemical properties of Nd Mg Ni-based alloys. Int J Hydrogen Energy 2017;42:19148e55. https://doi.org/10.1016/j.ijhydene.2017.06.170.
[54] Zuleta AA, Correa E, Sepúlveda M, Guerra L, Casta~ no JG, Echeverría F, et al. Effect of NH4HF2on deposition of alkaline electroless Ni-P coatings as a chromium-free pre-treatment for magnesium. Corros Sci 2012;55:194e200. https://doi.org/10.1016/j.corsci.2011.10.028.
[55] Sudagar J, Lian J, Sha W. Electroless Nickel, Alloy, Composite and Nano Coatingse A critical review, vol. 571; 2013. p. 183e204. https://doi.org/ 10.1002/chin.201331215.
[56] Chen X-B, Easton MA, Birbilis N, Yang H-Y, Abbott TB. Corrosion-resistant electrochemical plating of magnesium (Mg) alloys. In: Corros. Prev. Magnes. Alloy. Elsevier; 2013. p. 315e46. https://doi.org/10.1533/ 9780857098962.3.315.
[57] Ren Y, Zhou GS, Chai DL, Qiao XP. On the formation of the interlayer between Ni-P coating and AZ33 magnesium alloy substrate by means of in situ SEM observation. Chin Chem Lett 2014;25:947e52. https://doi.org/10.1016/ j.cclet.2014.04.009.
[58] Salman SA, Okido M. Anodization of magnesium (Mg) alloys to improve corrosion resistance. In: Corros. Prev. Magnes. Alloy.,. Elsevier; 2013. p. 197e231. https://doi.org/10.1533/9780857098962.2.197.
[59] Zhao H, Huang Z, Cui J. A new method for electroless Ni-P plating on AZ31 magnesium alloy. Surf Coating Technol 2007;202:133e9. https://doi.org/ 10.1016/j.surfcoat.2007.05.001.
[60] Jin JG, Lee SK, Kim YH. Adhesion improvement of electroless plated Ni layer by ultrasonic agitation during zincating process. Thin Solid Films 2004;466: 272e8. https://doi.org/10.1016/j.tsf.2004.02.100.
[61] Gao Y, Liu C, Fu S, Jin J, Shu X, Gao Y. Electroless nickel plating on ZM6 (Mg- 2.6Nd-0.6Zn-0.8Zr) magnesium alloy substrate. Surf Coating Technol 2010;204:3629e35. https://doi.org/10.1016/j.surfcoat.2010.04.040.
[62] Shao Z, Cai Z, Hu R, Wei S. The study of electroless nickel plating directly on magnesium alloy. Surf Coating Technol 2014;249:42e7. https://doi.org/ 10.1016/j.surfcoat.2014.03.043.
[63] Mallory GO. The electroless nickel plating bath: effect of variables on the process. Electroless Plat Fundam Appl 2009:69. 71; 72.
[64] Wang Z-C, Yu L, Qi Z-B, Song G-L. Electroless nickel-boron plating to improve the corrosion resistance of magnesium (Mg) alloys. In: Corros. Prev. Magnes. Alloy. Elsevier; 2013. p. 370e92. https://doi.org/10.1533/ 9780857098962.3.370.
[65] Ansari MI, Thakur DSG. Improvement of wear resistance of AZ91 magnesium alloy by electroless Ni-P coatings with in uence of surfactant. Mater Today Proc 2017;4:9870e4. https://doi.org/10.1016/j.matpr.2017.06.284.
[66] Wu L, Yang Z, Qin G. Kinetic study of a novel electroless Ni P deposition on AZ91D magnesium alloy using nickel hypophosphite as the metal salt. J Alloy Comp 2017;694:1133e9. https://doi.org/10.1016/j.jallcom.2016.10.141.
[67] Lian JS, Li GY, Niu LY, Gu CD, Jiang ZH, Jiang Q. Electroless NieP deposition plus zinc phosphate coating on AZ91D magnesium alloy. Surf Coating Technol 2006;200:5956e62. https://doi.org/10.1016/j.surfcoat.2005.09.007.
[68] Jin J, Liu C, Fu S, Gao Y, Shu X. Electroless Ni-P plating on Mg-10Gd-4.8Y- 0.6Zr magnesium alloy with a new pretreatment process. Surf Coating Technol 2011;206:348e53. https://doi.org/10.1016/j.surfcoat.2011.07.023. 644 A. Thakur et al. / Defence Technology 15 (2019) 636e644
[69] Imran M, Dineshsingh A. A high performance of ENie P coatings on me- chanical and tribological properties with in uence of bath pH on AZ91 magnesium alloy. J Fail Anal Prev 2016;17:100e6. https://doi.org/10.1007/ s11668-016-0210-2.
[70] Seifzadeh D, Rajabalizadeh Z. Surface & Coatings Technology Environmentally-friendly method for electroless Nie P plating on magne- sium alloy. Surf Coating Technol 2013;218:119e26. https://doi.org/10.1016/ j.surfcoat.2012.12.039.
[71] Song YW, Shan DY, Han EH. Corrosion behaviors of electroless plating NieP coatings deposited on magnesium alloys in arti cial sweat solution. Elec- trochim Acta 2007;53. https://doi.org/10.1016/j.electacta.2007.08.062. 2009e15.
[72] Cheong WJ, Luan BL, Shoesmith DW. Protective coating on Mg AZ91D alloy - the effect of electroless nickel (EN) bath stabilizers on corrosion behaviour of Ni-P deposit. Corros Sci 2007;49:1777e98. https://doi.org/10.1016/ j.corsci.2006.08.025.
[73] Iranipour N, Khosroshahi RA, Ahmadi NP. Surface & Coatings Technology A study on the electroless Nie P deposition on WE43 magnesium alloy. Surf Coating Technol 2010;205:2281e6. https://doi.org/10.1016/ j.surfcoat.2010.09.006.
[74] Ambat R, Zhou W. Electroless nickel-plating on AZ91D magnesium alloy: effect of substrate microstructure and plating parameters. Surf Coating Technol 2004;179:124e34. https://doi.org/10.1016/S0257-8972(03)00866- 1.
[75] Correa E, Zuleta AA, Guerra L, Gomez MA, Casta~ no JG, Echeverría F, et al. Tribological behavior of electroless NieB coatings on magnesium and AZ91D alloy. Wear 2013;305:115e23. https://doi.org/10.1016/j.wear.2013.06.004.
[76] Anik M, Akay I, Topcu S. Effect of electroless nickel coating on the electro- chemical hydrogen storage characteristics of Al and Zr including Mg-based alloys. Int J Hydrogen Energy 2009;34:5449e57. https://doi.org/10.1016/ j.ijhydene.2009.05.029.
[77] Zhang WX, Jiang ZH, Li GY, Jiang Q, Lian JS. Electroless Ni-P/Ni-B duplex coatings for improving the hardness and the corrosion resistance of AZ91D magnesium alloy. Appl Surf Sci 2008;254:4949e55. https://doi.org/10.1016/ j.apsusc.2008.01.144.
[78] Narayanan TSNS, Krishnaveni K, Seshadri SK. Electroless Ni-P/Ni-B duplex coatings: preparation and evaluation of microhardness, wear and corrosion resistance. Mater Chem Phys 2003;82:771e9. https://doi.org/10.1016/ S0254-0584(03)00390-0.
[79] Bonin L, Vitry V. Mechanical and wear characterization of electroless nickel mono and bilayers and high boron-mid phosphorus electroless nickel duplex coatings. Surf Coating Technol 2016;307:957e62. https://doi.org/10.1016/ j.surfcoat.2016.10.021.
[80] Zhang WX, Huang N, He JG, Jiang ZH, Jiang Q, Lian JS. Electroless deposition of NieWeP coating on AZ91D magnesium alloy. Appl Surf Sci 2007;253: 5116e21. https://doi.org/10.1016/j.apsusc.2006.11.022.
[81] Balaraju JN, Rajam KS. Electroless deposition of Ni-Cu-P, Ni-W-P and Ni-W- Cu-P alloys. Surf Coating Technol 2005;195:154e61. https://doi.org/10.1016/ j.surfcoat.2004.07.068.
[82] Narayanan TSNS, Selvakumar S, Stephen A. Electroless Ni-Co-P ternary alloy deposits: preparation and characteristics. Surf Coating Technol 2003;172: 298e307. https://doi.org/10.1016/S0257-8972(03)00315-3.
[83] Kim BC, Innis PC, Wallace GG, Low CTJ, Walsh FC, Cho WJ, et al. Electrically conductive coatings of nickel and polypyrrole/poly(2-methoxyaniline-5- sulfonic acid) on nylon Lycra® textiles. Prog Org Coating 2013;76:1296e301. https://doi.org/10.1016/j.porgcoat.2013.04.004.
[84] Palaniappa M, Seshadri SK. Friction and wear behavior of electroless Ni-P and Ni-W-P alloy coatings. Wear 2008;265:735e40. https://doi.org/10.1016/ j.wear.2008.01.002.
[85] Shibli SMA, Chinchu KS. Development and electrochemical characterization of Ni-P coated tungsten incorporated electroless nickel coatings. Mater Chem Phys 2016. https://doi.org/10.1016/j.matchemphys.2016.04.034.
[86] Selvi VE, Chatterji P, Subramanian S, Balaraju JN. Surface & Coatings Technology Autocatalytic duplex Nie P/Nie We P coatings on AZ31B magnesium alloy. Surf Coating Technol 2014;240:103e9. https://doi.org/ 10.1016/j.surfcoat.2013.12.022.
[87] Shu X, Wang Y, Liu C, Aljaafari A, Gao W. Surface & Coatings Technology Double-layered Ni-P/Ni-P-ZrO 2 electroless coatings on AZ31 magnesium alloy with improved corrosion resistance. Surf Coating Technol 2015;261: 161e6. https://doi.org/10.1016/j.surfcoat.2014.11.040.
[88] Heshmati M, Seifzadeh D, Shoghi P, Gholizadeh-Gheshlaghi M. Duplex Ni- Zn-Cu-P/Ni-P electroless coating on magnesium alloy via maleic acid pre- treatment. Surf Coating Technol 2017;328:20e9. https://doi.org/10.1016/ j.surfcoat.2017.08.027.
[89] Gan R, Wang D, Xie Z, He L. Improving surface characteristic and corrosion inhibition of coating on Mg alloy by trace stannous (II) chloride. Corros Sci 2017;123:147e57. https://doi.org/10.1016/j.corsci.2017.04.018.
[90] Wang H, Liu L, Dou Y, Zhang W, Jiang W. Applied Surface Science Preparation and corrosion resistance of electroless Ni-P/SiC functionally gradient coat- ings on AZ91D magnesium alloy. Appl Surf Sci 2013;286:319e27. https:// doi.org/10.1016/j.apsusc.2013.09.079.
[91] Firoozbakht M, Monirvaghe SM, Niroumand B. Electroless composite coating of Nie Pe carbon nanotubes on magnesium powder. J Alloy Comp 2011;509:S496e502. https://doi.org/10.1016/j.jallcom.2011.02.023.
[92] Ansari MI, Thakur DG. In uence of surfactant: using electroless ternary nanocomposite coatings to enhance the surface properties on AZ91 mag- nesium alloy. Surf Interf 2017;7:20e8. https://doi.org/10.1016/ j.sur n.2017.02.004.
[93] Chen W, Gao W, He Y. Surface & Coatings Technology A novel electroless plating of Nie Pe TiO 2 nano-composite coatings. Surf Coating Technol 2010;204:2493e8. https://doi.org/10.1016/j.surfcoat.2010.01.032.
[94] Yang S, Liu H, Han S, Li Y, Shen W. Applied Surface Science Effects of elec- troless composite plating Nie Cue P on the electrochemical properties of La e Mge Ni-based hydrogen storage alloy. Appl Surf Sci 2013;271:210e5. https://doi.org/10.1016/j.apsusc.2013.01.161.
[95] Jiang BL, Ge YF. Micro-arc oxidation (MAO) to improve the corrosion resis- tance of magnesium (Mg) alloys. In: Corros. Prev. Magnes. Alloy. Elsevier; 2013. p. 163e96. https://doi.org/10.1533/9780857098962.2.163.
[96] Sun S, Liu J, Yan C, Wang F. A novel process for electroless nickel plating on anodized magnesium alloy. Appl Surf Sci 2008;254:5016e22. https://doi.org/ 10.1016/j.apsusc.2008.01.169.
[97] Husain EAM, Husain A, Abdel Nazeer A, Al-Saraf JM, Al-Awadhi K, Shekeban A, et al. Behavior of electrodeposited layer on Mg (ZE41A) alloy under tribo-corrosion condition. Int J Electrochem Sci 2015;10:5194e208.
[98] Murakami K, Hino M, Hiramatsu M, Nakai K, Kobayashi S, Saijo A, et al. Corrosion protection of AZ91D magnesium alloy by anodization using phosphate electrolyte. Mater Trans 2007;48:3101e8. https://doi.org/ 10.2320/matertrans.L-MRA2007881.
[99] Zhang RF, Zhang SF. Formation of micro-arc oxidation coatings on AZ91HP magnesium alloys. Corros Sci 2009;51:2820e5. https://doi.org/10.1016/ j.corsci.2009.08.009.
[100] Song Z, Xie Z, Yu G, Hu B, He X, Zhang X. A novel palladium-free surface activation process for electroless nickel deposition on micro-arc oxidation lm of AZ91D Mg alloy. J Alloy Comp 2015;623:274e81. https://doi.org/ 10.1016/j.jallcom.2014.10.130.
[101] Zeng L, Yang S, Zhang W, Guo Y, Yan C. Preparation and characterization of a double-layer coating on magnesium alloy AZ91D. Electrochim Acta 2010;55: 3376e83. https://doi.org/10.1016/j.electacta.2010.01.041.
[102] Ezhilselvi V, Balaraju JN, Subramanian S. Chromate and HF free pretreatment for MAO/electroless nickel coating on AZ31B magnesium alloy. Surf Coating Technol 2017;325:270e6. https://doi.org/10.1016/j.surfcoat.2017.06.049.
[103] Jian SY, Lee JL, Lee H Bin, Sheu HH, Ou CY, Ger M Der. In uence of electroless plating on the deterioration of the corrosion resistance of MAO coated AZ31B magnesium alloy. J Taiwan Inst Chem Eng 2016;68:496e505.



































