A plater wrote to me in regard to recently experiencing some serious corrosion failures that were found to be caused by “macro-cracks” in the chromium.
 Frank AltmayerThey didn’t know what caused cracking in chromium and asked for my assistance.,
Frank AltmayerThey didn’t know what caused cracking in chromium and asked for my assistance.,
Weʼll start with the basics: chromium that is plated from a conventional hexavalent chromium plating solution under “normal” conditions can be expected to crack on a microscopic basis. As the thickness builds, additional cracks are produced, but they tend not to line up with each other, resulting in a random pattern of cracking, with each crack at a limited level of depth.
Depending on plating conditions and the chemical make-up of the plating solution, several mils of thickness may be required before all cracks are covered to an extent that there is no path from the top down to the bottom of the chromium layer. Whether there is a horizontal path through the chromium, which would result in corrosion failure, would be dependent upon the depth of the cracks at the surface, the width of those cracks and the crack density.
For example, if the crack density is high, the depth is low and the crack width is narrow, we would expect better corrosion performance than if the opposite conditions were present.
Chromium Hydrides
 Cross section and crack pattern of a normal hard chromium deposit (photomicrograph at 400x).Researchers (example, Snavely, Trans. of the Electrochem Society, 1947, 92, p. 527–577) have concluded that the cracking is produced by the formation of unstable chromium hydrides. Chromium hydrides have crystal structures significantly different in volume from pure chromium, which is body-centered cubic (bcc). Hydrides (examples are CrH, CrH2, Cr2H) can take either a face-centered cubic structure (fcc) or a hexagonal structure.
Cross section and crack pattern of a normal hard chromium deposit (photomicrograph at 400x).Researchers (example, Snavely, Trans. of the Electrochem Society, 1947, 92, p. 527–577) have concluded that the cracking is produced by the formation of unstable chromium hydrides. Chromium hydrides have crystal structures significantly different in volume from pure chromium, which is body-centered cubic (bcc). Hydrides (examples are CrH, CrH2, Cr2H) can take either a face-centered cubic structure (fcc) or a hexagonal structure.
Under most normal plating conditions, the hexagonal structure is favored. Chromium tied to hydrogen in a hexagonal structure takes up more space than chromium all by itself. The chromium hydride is not stable and breaks down to form chromium metal.
The process of the formation of chromium hydrides, and the break-down of the hydrides to chromium metal, takes place during plating, and can also take place, to some extent, for days after plating. As the hydride breaks down, the deposit shrinks in volume by up to 15 percent. This is sort of like wet mud drying and shrinking in volume. The result is mud-cracking on a microscopic scale. The size, number, shape and depth of the microcracks is dependent upon:
- Variation in hardness/stress of the basis metal
- Quality of rectification (ripple)
- Plating conditions such as temperature, current density, agitation
- Plating solution purity, including heavy metal content, trivalent content, and chloride content
- Solution ratio (chromic acid to sulfate)
- Temperature gradients after plating
- Grinding/polishing technique after plating
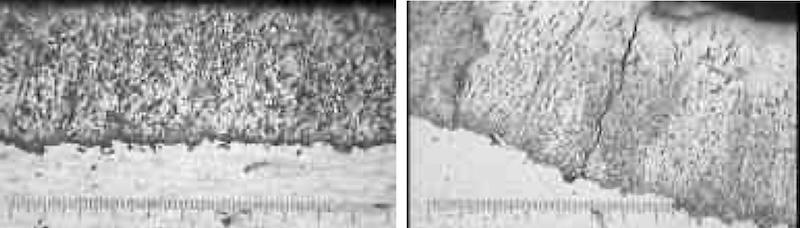 At left, cross section and crack pattern of a thin-dense hard chromium deposit (photomicrograph at 400x). At right, cross section of undesirable macro crack in hard chromium deposit (photomicrograph at 400x).
At left, cross section and crack pattern of a thin-dense hard chromium deposit (photomicrograph at 400x). At right, cross section of undesirable macro crack in hard chromium deposit (photomicrograph at 400x).
During the plating process, a layer of chromium-chromium hydride is deposited and decomposes to chromium, resulting in cracks. The cracks are bridged as plating progresses, eventually covering all of the cracks produced at a given depth. Then the process is repeated, with the newly formed cracks being bridged by the newly deposited chromium-chromium hydride.
The result is micro-cracks that generally do not line up with each other as seen in photos of cross sections we have provided.
Macro-cracking such as what the plater described can be the result of poor control over any or all of the seven items listed here.
Frank Altmayer is a Master Surface Finisher, an AESF Fellow, and the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in metal finishing. He received the AESF Past Presidents Award, NAMF Award of Special Recognition, AESF Leadership Award, AESF Fellowship Award, Chicago Branch AESF Geldzahler Service Award, and NASF Award of Special Recognition.



































