According to the Bureau of Safety and Environmental Enforcement and the Bureau of Ocean Energy), there are over 1,800 and 2,000 oil and gas offshore platforms and coastal facilities in the Gulf of Mexico alone.
 From top left, Steve Cabral, Oscar Garcia, Jonathan L. McCrea, Leo Monaco, Mioara Neacsu, and Gino Palumbo.Steel fasteners are used extensively throughout these platforms and are critical on numerous components, including pressure boundary and wellhead control devices. The likelihood of a fastener failure is directly related to both the mechanical strength of the fastener in the operational environment and the service range stresses, neither of which remain constant over time due to the highly corrosive environment of the offshore platform.
From top left, Steve Cabral, Oscar Garcia, Jonathan L. McCrea, Leo Monaco, Mioara Neacsu, and Gino Palumbo.Steel fasteners are used extensively throughout these platforms and are critical on numerous components, including pressure boundary and wellhead control devices. The likelihood of a fastener failure is directly related to both the mechanical strength of the fastener in the operational environment and the service range stresses, neither of which remain constant over time due to the highly corrosive environment of the offshore platform.
Critical bolting is inspected, replaced, and maintained annually to prevent corrosion-related failures. On average, an offshore platform replaces over 70% of the bolting yearly. Six offshore platforms owned by one major integrated oil and gas company use over 175,000 lbs of carbon steel fasteners annually. Bolting lifespans must be increased to minimize replacement frequency, saving cost, and downtime.
In this whitepaper, ZNnGard, a registered trademark product, is an ideal solution for improving the corrosion performance of high-pressure steel bolting used in valves, flanges, piping, etc.
Conventional Corrosion Protection Coatings
Conventional corrosion protection coatings improve the corrosion resistance of a steel fastener and can often be grouped into two categories: barrier coatings and sacrificial coatings. Barrier coatings often have very high corrosion resistance and protect the steel by providing a physical barrier between the steel and the corrosive environment. Sacrificial coatings, as the name implies, will preferentially corrode while cathodically protecting the underlying steel substrate. Still, they are limited to certain metals that act as the galvanic anode when coupled to steel (e.g., Zn, ZnNi, Al). Sacrificial coatings have the added benefit of being able to protect the steel even when there is a breach in the coating (e.g., from scratches, bolt torquing, holidays/defects in the coating during application); as such, they can often provide superior protection in aggressive environments.
Cadmium-plated studs and nuts were used extensively from the 1970s to the late 1980s. They provided good corrosion resistance as a sacrificial coating with good lubricity and a relatively long life span. Cadmium, however, is a toxic heavy metal that is harmful to human health. The Environmental Protection Agency lists Cd as a probable human carcinogen (class B1)¹. It strictly regulates the emissions of Cd to the atmosphere and the disposal of Cd and Cd-containing wastes. In addition, cadmium is not biodegradable, which means it can remain in the environment for a long time after it is released, causing long-term damage to the ecosystem. The potential for bioaccumulation of cadmium in marine environments is a concern. It can result in high concentrations in the tissues of marine life, such as fish and other animals, if not properly managed.
Over the last few decades, the oil and gas, aerospace, and defense industries have investigated multiple Cd-alternate coating technologies for steel fasteners. Some of the most promising concepts are starting to be accepted in various military and commercial Cd-replacement roles. However, to date, there is no universal solution.
Zinc (galvanized or electroplated) and zinc-nickel (electroplated) are two of the most promising alternatives, often finished with a topcoat (e.g., chromate conversion coatings, fluoropolymer). Fluoropolymer topcoats are barrier coatings that provide low friction and consistent torque, but torquing during installation can easily damage the coating. One of the major disadvantages of fluoropolymers, however, is their environmental persistence. These materials do not break down easily in the environment and can accumulate in the food chain, potentially leading to health problems for wildlife and humans. Additionally, producing fluoropolymers can release perfluorinated compounds (PFCs) into the air, harming human health and the environment. Because of these concerns, the use of fluoropolymers has come under increased scrutiny in recent years.
Like cadmium, traditional direct current (DC) electroplated zinc and zinc-nickel coatings act as a sacrificial coating to protect the steel. Due to the more active nature of zinc coatings (larger potential difference between zinc and steel), they corrode more quickly than cadmium and require frequent bolting replacement. Several groups have identified DC electroplated ZnNi as offering properties that make it promising as a Cd alternative. However, one of the shortfalls² is related to the potential for hydrogen embrittlement and re-embrittlement of high-strength steels during exposure to a corrosive environment such as salt water, which is an environment of particular interest for the U.S. Navy (specifically for critical load-bearing components such as wheels and landing gear) and for offshore oil platforms with the use of high strength steel fasteners.
To alleviate the issues with hydrogen embrittlement, DC-plated zinc-nickel formulations have been created with intentional porosity to allow the hydrogen generated in the plating process to escape during post-plating hydrogen relief baking. However, the porous coating resulted in questionable hydrogen re-embrittlement performance if the ZnNi coating did not have a subsequent polymeric top coat. DC-plated zinc-nickel coatings have also been found to require higher torque values to achieve the same tension values as cadmium due to the higher roughness associated with the porous nature of the coating.
Ceramic-metallic coatings followed by a fluoropolymer topcoat are also a common alternative but are beginning to decline in use. This system has better corrosion resistance and a consistent friction effect; however, the ceramic coatings are thick (>1 mil) and require oversized nut tapping to accommodate the coating thickness.
At the direction of BSEE, the industrial standards that set the requirements for allowance and tolerance of the bolt and nuts threads have recently changed. The American Petroleum Institute Standard API-20E, “Alloy and Carbon Steel Bolting for Use in the Petroleum and Natural Gas Industries,” specifies that “Oversizing of nut threads or under-sizing of bolt threads is not permissible.” However, ASME B1.1 Unified Inch Screw Threads Class 2A fit (B1.13M Class 6g fit Metric) specifies, “Unless otherwise specified, size limits for standard external thread Class 2A apply before coating. The external thread allowance may thus accommodate the coating thickness on coated parts, provided that the maximum coating thickness is no more than one-fourth of the allowance.”
While the ASME standard is accepted, it directly contradicts the API specification and the BSEE guidelines. Regardless, a corrosion coating system that does not require oversized nuts is preferred.
Nanostructured ZnNi for Cd Replacement
Recognizing the need to replace cadmium on fasteners and a range of manufactured components, the Strategic Environmental Research and Development Program (SERDP) funded a program with Integran Technologies Inc. on cadmium replacement using electrodeposited nanocrystalline zinc-nickel alloy coatings. SERDP project WP-1616 investigated pulse plating to obtain a fine-grained microstructure using different Zn-based electroplating chemistries for potential cadmium replacement. Through this work, a new electroplated ZnNi alloy was developed utilizing pulse plating of alkaline-based plating chemistry that provided excellent performance and a clear benefit from using pulse plating compared to traditional DC plating.
The fine-grained structures produced via pulse plating from modified commercial ZnNi-alloy plating solutions were found to have several benefits over conventional DC plating, including bright, uniform, dense microstructures; uniform, equiaxed grain size throughout the thickness of the coating; increased microhardness; single γ-phase crystallographic microstructure; increased corrosion resistance compared to other Zn-Ni alloys; and decreased friction. They also passed the ASTM F519 hydrogen embrittlement (HE) test. ZnNi coatings synthesized from alkaline bath chemistries had greater compositional uniformity (less fluctuation with current density variations) than those from acid chemistries, resulting in a more uniform peak-to-valley composition in threaded fasteners. In addition to providing dense structures, pulse-plated deposits typically possessed higher nickel content than DC-plated deposits from the same plating bath. Both Cr6+ and Cr3+ conversion coatings were successfully applied to the pulse-plated ZnNi structures, enhancing salt spray corrosion performance similarly. The ability to pass the HRE test was highly dependent on the following factors: pre-plating treatment methods, porosity of coating, nickel content, and the open circuit potential (OCP). Finally, bulk processing of large numbers of fasteners was accomplished using pulsed electrical parameters while barrel plating.
Sigma Fasteners believes pulse-plated Nanostructured zinc nickel-coated fasteners will become widely accepted as the long-sought replacement for cadmium-plated bolts and PTFE formerly used in Oil and Gas industries worldwide. It recently licensed the pulse-plated Nanostructured ZnNi technology from Integran Technologies. Dipsol America is the master distributor for the expressly branded ZNnGard chemistry and components for plating and chromate finish in the oil and gas sector. A ZNnGard production line for rack and barrel plating steel fasteners has been installed at Sigma Fasteners’ facilities in Houston, Texas.
Figure 1 shows optical micrographs of a cross-section of conventional DC electroplated ZnNi coated onto steel (left image) and compares it to pulse-plated ZNnGard coated onto steel (right image), both coatings produced using the same alkaline bath chemistry. Using the pulse plating waveforms with the alkaline ZnNi plating system led to a uniform dense microstructure compared to the porous microstructure that resulted from DC plating. Due to the high diffusivity of hydrogen through the nanocrystalline grain structure, the dense microstructure does not cause hydrogen embrittlement issues, and all test samples passed ASTM F519 hydrogen embrittlement testing; this is a different mechanism to relieve hydrogen compared to conventional DC electroplated ZnNi that relies on its porous microstructure.
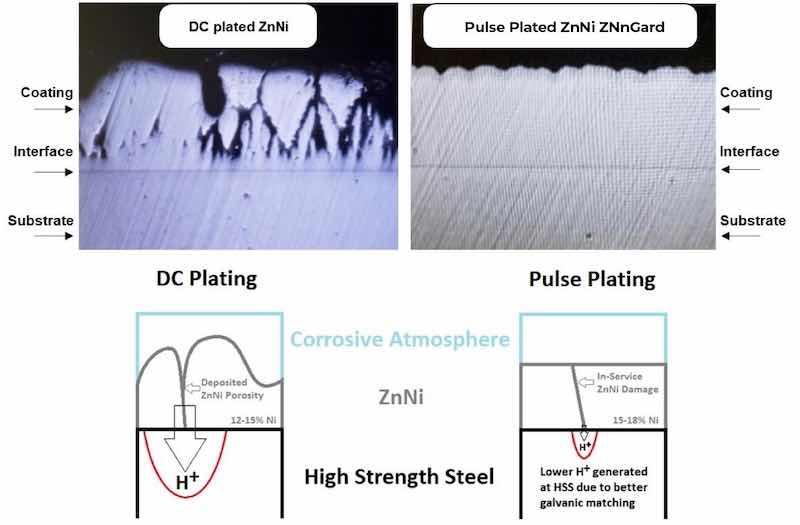
Figure 1: Optical micrograph of a conventional DC electroplated ZnNi coating on steel (top left) and a pulse-plated ZnNi (ZNnGard) coating on steel, synthesized from the same Alkaline bath chemistry. A schematic (bottom) illustrating how porosity in a ZnNi deposit can increase the probability of hydrogen diffusing into the steel substrate during corrosion.
The resulting dense microstructure also provides superior protection against hydrogen re-embrittlement (a.k.a in-service embrittlement) by reducing the probability of hydrogen diffusing into the steel substrate during active corrosion. Resistance to hydrogen re-embrittlement is particularly important as it is a critical parameter for evaluating the practical use of high-strength steel fasteners, and it is a difficulty with many of the current cadmium-replacement alloys. The uniform, fully dense nanocrystalline microstructure improves hardness and wear resistance, lowers friction coefficients, and improves aesthetics.
Corrosion Protection of Steel using ZNnGard
ZNnGard provides excellent corrosion protection for steel fasteners and bolts in offshore oil and gas environments, prolonging their in-service life. The corrosion performance is attributed to its microstructure and composition (uniform, dense, nanostructured, single γ-phase). ZNnGard acts as a sacrificial coating to steel, protecting the steel even if the coating is damaged due to its lower (more negative) OCP compared to steel (ZNnGard acts as the anode, preferentially corroding while the steel acts as the cathode and is protected).
Figure 2 provides a comparison of the OCP of ZNnGard (including a trivalent chromate topcoat) and some other coatings in salt water (5% NaCl); the OCP is commonly used as a method to predict the galvanic corrosion effects of materials (in this case a lower OCP than steel is required for cathodic protection). As shown in Figure 2, ZNnGard has a lower OCP than steel, but not as low as conventional zinc coatings; this is preferable since the smaller OCP difference reduces the polarization of steel when exposed through the coating (lower driving force for hydrogen re-embrittlement of the steel). ZNnGard has also shown an order-of-magnitude lower corrosion rate than conventional zinc in aqueous salt water (Figure 2), suggesting a longer lifespan for ZNnGard.
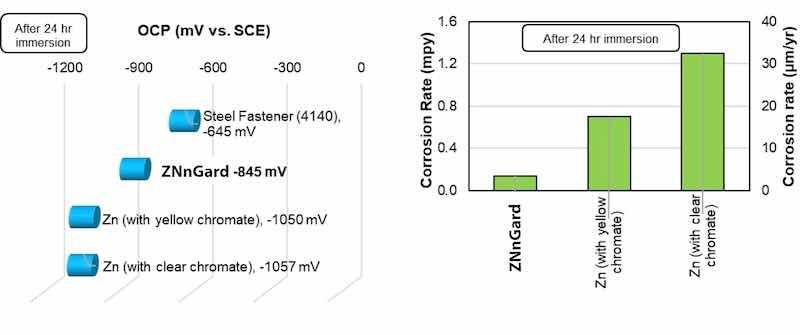
Figure 2: The corrosion performance of ZNnGard is compared to other materials, showing the OCP (left) and corrosion rate (right). ZNnGard includes a trivalent chromate topcoat.
The corrosion protection of steel using ZNnGard (including a trivalent chromate topcoat) was demonstrated using salt spray testing following a modified ASTM B117 procedure, with the results shown in Figure 3. Figure 3 presents three sets of salt spray testing data. The top data set in Figure 3 compares the scribed salt spray performance of steel Q-panels coated with ZNnGard to those electroplated with conventional zinc coatings (with trivalent chromate); ZNnGard outperforms the conventional zinc coatings with ZNnGard showing no indications of red nor white rust. The middle data set in Figure 3 shows the long-term salt spray corrosion performance of scribed ZNnGard coated steel panels; no red rust is observed even up to ~7200 hours. Finally, the bottom data in Figure 3 presents the salt spray testing of ZNnGard-coated steel bolts that showed no red rust up to ~8000 hours.
Summary
ZNnGard is a dense, nanostructured ZnNi alloy coating that is produced via pulse electrodeposition using an alkaline Zn-Ni bath chemistry and can lead to the retention of numerous benefits associated with Cd-coating technology, including non-line-of-sight application, excellent corrosion resistance, low coefficient of friction, excellent coating adhesion, high dimensional consistency, and superior surface finish. Pulse-plated ZnnGard was also beneficial over conventional DC ZnNi electroplating plating, including superior corrosion protection and improved HRE performance. Pulse electrodeposition can be implemented within the existing Cd-plating infrastructure for conventional rack plating and bulk plating of fasteners, e.g., barrel plating.
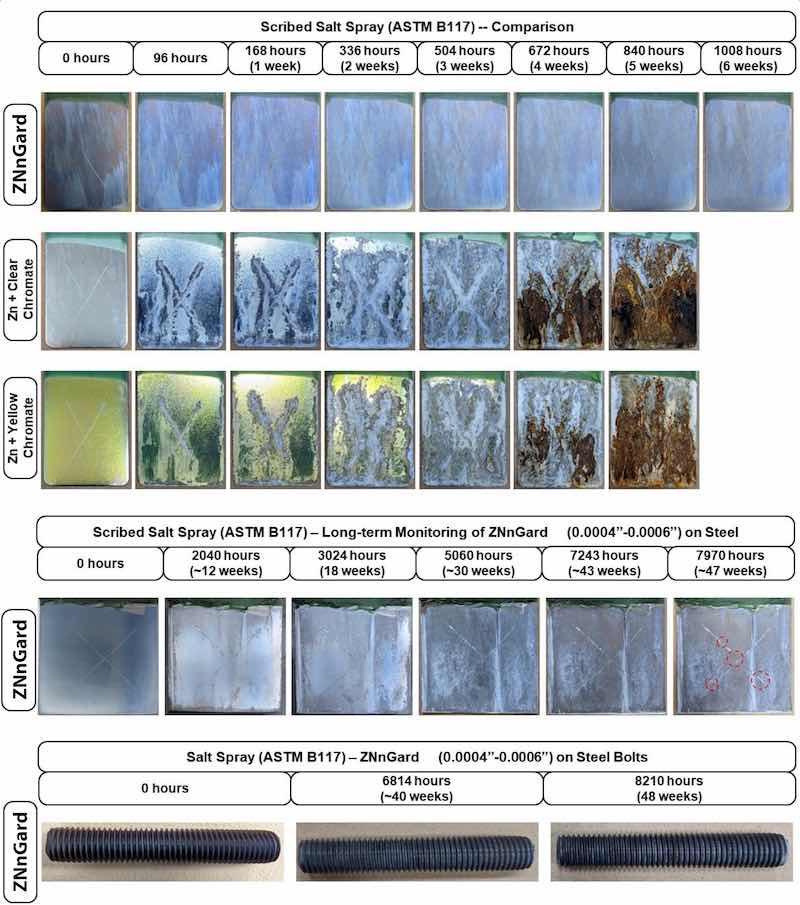
Figure 3: The salt spray (ASTM B117) corrosion performance of ZNnGard-coated steel panels is compared to conventional electroplated Zn with a Clear or Yellow Chromate coating (top set of images). ZNnGard includes a trivalent chromate topcoat. Samples were scribed before testing using a Lathe Tool Type as per ASTM D1654. Salt spray testing was monitored for up to ~1000 hours, showing no red or white rust on ZNnGard, while the Zn samples showed extensive white and red rust. Long-term salt spray performance of ZNnGard (middle set of images) and ZNnGard coated onto a steel bolt (bottom set of images) are also provided.
Steve Cabral and Oscar Garcia are with Sigma Fasteners; Jonathan L. McCrea, Leo Monaco, Mioara Neacsu, and Gino Palumbo are with Integran Technologies. Visit www.sigmafasteners.com and www.integran.com.
References
- http://www.epa.gov/ttn/atw/hlthef/cadmium.html (accessed February 2012).
- For example, see USAF/ES3/Boeing Presentation, “Low Hydrogen Embrittlement (LHE) zinc-nickel (Zn-Ni) Qualification Test Result and Process Parameters Development,” (9 February 2011).



































