The corrosion resistance of tin/zinc alloys is investigated by EC, CMT, ASTM B117 and Prohesion tests in this edited version of the Garland Award winner from the 2000 Aerospace/Airline Plating & Metal Finishing Forum.
 Dr. RasmussenThe optimal composition of alloying zinc is 20–25 wt-%. A new proprietary process for DC deposition of tin/zinc coating with optimal composition has been developed, and the alloy composition is not influenced by the applied current density.
Dr. RasmussenThe optimal composition of alloying zinc is 20–25 wt-%. A new proprietary process for DC deposition of tin/zinc coating with optimal composition has been developed, and the alloy composition is not influenced by the applied current density.
Both tin and zinc are widely used to protect steel against corrosion. Each has a different range of applications, and they protect steel by different mechanisms. Tin is nobler than steel and, under ordinary atmospheric exposure, protects steel by forming a corrosion-resistant envelope around it. However, rusting takes place in pinholes or imperfections in the tin coating and is accelerated galvanically by the difference in potential between steel and tin. Zinc is less noble than steel and is protected by sacrificial action. Even when steel is exposed through faults in the coating, it is protected galvanically by zinc.
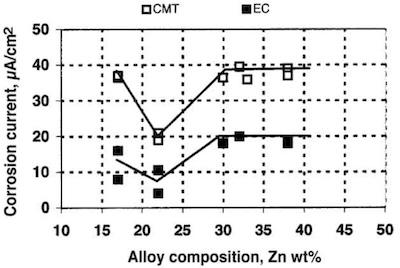 Fig. 1—Corrosion current vs. alloy composition. Unpassivated coatings: pH=6.5000, and dissolved oxygen is present.The protective properties of the two metals are nicely balanced in a tin zinc alloy containing about 75–80 percent tin. The coating protects steel by a sacrificial action similar to that of zinc. Consequently, steel does not rust through pinholes, and yet the coating does not form as voluminous white corrosion products as with zinc coatings. A tin/zinc coating is crack-free and has been reported to do better than zinc coatings during moist SO2 testing.
Fig. 1—Corrosion current vs. alloy composition. Unpassivated coatings: pH=6.5000, and dissolved oxygen is present.The protective properties of the two metals are nicely balanced in a tin zinc alloy containing about 75–80 percent tin. The coating protects steel by a sacrificial action similar to that of zinc. Consequently, steel does not rust through pinholes, and yet the coating does not form as voluminous white corrosion products as with zinc coatings. A tin/zinc coating is crack-free and has been reported to do better than zinc coatings during moist SO2 testing.
Alloy compositions outside the above range result in reduced corrosion resistance, especially if the tin content is higher than 80 percent. The overall corrosion performance is highly dependent on the relation between alloy composition and current density. Currently, electrochemical processes for the deposition of tin/zinc coating show high amounts of tin in low current density areas and high amounts when the current density is high.
What follows describes results from corrosion testing of tin/zinc alloys and a new proprietary process for deposition of tin/zinc alloys with optimal composition over a large range of current densities.
Table 1: ASTM B117 Corrosion Testing of Tin/Zinc Alloys
| Zinc Wt-% | Thickness µm | Chromate | Time to red rust 1% coverage |
| 20-22 | 10-1 | Yes | 1512h |
| 20-22 | 10-11 | Yes | 1536h |
| 20-22 | 10-11 | No | 816h |
| 20-22 | 10-11 | No | 792h |
| 20-22 | 10-11 | No | 816h |
Corrosion Protection
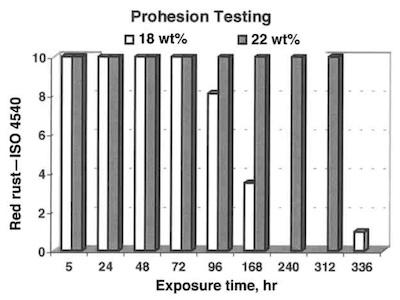 Fig. 2—Propagation of red rust according to ISO 4540 for 5 μm thick coatings.The corrosion resistance of electrodeposited tin/zinc alloys is determined in accelerated laboratory tests. The investigation comprises neutral salt spray (ASTM B117) and Prohesion tests. The amount of rust is assessed according to ISO 4540.
Fig. 2—Propagation of red rust according to ISO 4540 for 5 μm thick coatings.The corrosion resistance of electrodeposited tin/zinc alloys is determined in accelerated laboratory tests. The investigation comprises neutral salt spray (ASTM B117) and Prohesion tests. The amount of rust is assessed according to ISO 4540.
The Prohesion test is an accelerated corrosion test. It has been reported that this test relates more closely to long-term natural exposures than conventional salt spray tests. The test uses an electrolyte of 0.4 percent ammonium sulfate and 0.05 percent sodium chloride. A cycle consists of one hr spray at ambient temperature and one hr drying at 35C (95F).
The corrosion current is measured electrochemically (EC), and the corrosion measurements are measured using the titration (CMT) method.1 The general condition for applying the CMT method is the anodic metal dissolution reaction:
Me → Men+ + n˙e-
combines with one of the following cathodic reactions:
n˙H+ + n˙e- → (n/2)˙H2
or
(n/4) ˙O2 + n˙H+ + n˙e- → (n/2)˙H2O
so that a net consumption of acid takes place, equivalent to the number of electric charges (flowing as the corrosion current) and to the number of metal atoms dissolving.
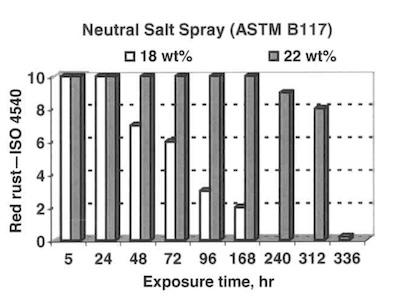 Fig. 3—Propagation of red rust according to ISO 4540 for 5 μm thick coatings.The CMT and EC measurements show that the corrosion current for unpassivated alloys in a 3-percent sodium chloride solution at pH=6.5 has a minimum when the alloy contains 20–25 wt-% of zinc (Fig. 1).
Fig. 3—Propagation of red rust according to ISO 4540 for 5 μm thick coatings.The CMT and EC measurements show that the corrosion current for unpassivated alloys in a 3-percent sodium chloride solution at pH=6.5 has a minimum when the alloy contains 20–25 wt-% of zinc (Fig. 1).
The corrosion performance of tin/ zinc alloys in accelerated laboratory tests (ASTM B117 and Prohesion) is show in Figs. 2 and 3 for optimal alloy composition and for coatings with a higher amount of tin (outside optimal range). Both tests show a significant reduction in corrosion performance, if the amount of tin is higher than 20 percent.
Additional ASTM B117 testing of 10 µm thick chromated tin/zinc coatings with an optimal composition (78–80% tin) is shown in Table 1. The propagation of red rust, corresponding to 1 percent coverage (rating 6 in ISO 4540), takes approximately 1500 hr of corrosive exposure, which is significantly longer than the performance of zinc coatings. Compared to non-chromated coatings, the corrosion resistance is approximately doubled by applying a chromate post-treatment.
Deposition of Tin/Zinc Coatings
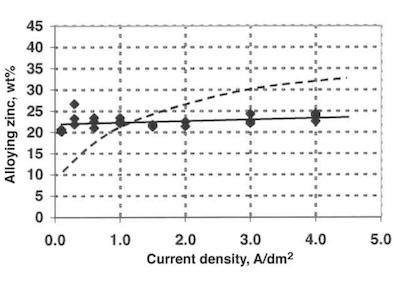 Fig. 4—Alloy composition vs. current density in the new tin/zinc process.The overall corrosion performance of tin/zinc coatings is highly dependent on the alloy composition. The overall corrosion performance of a complex-shaped part is related to the variation in alloy composition in areas plated under low (holes, recesses) or high (edges, protrusions) current densities. The performance, therefore, is dependent on the correlation between alloy composition and current density.
Fig. 4—Alloy composition vs. current density in the new tin/zinc process.The overall corrosion performance of tin/zinc coatings is highly dependent on the alloy composition. The overall corrosion performance of a complex-shaped part is related to the variation in alloy composition in areas plated under low (holes, recesses) or high (edges, protrusions) current densities. The performance, therefore, is dependent on the correlation between alloy composition and current density.
A new proprietary process for the deposition of tin/zinc alloy with an optimal composition of 20-25 percent zinc independent of current density (0.1 A/dm2 – 4 A/dm2) has been developed.
The new process is made up of tin and zinc sulfates, a complex agent, an additive system, and an antioxidant. The bath is neutral and operated at room temperature. The additive system developed results in uniform Hull cell appearance and suppresses the tin deposition mechanism, or enhances zinc deposition at low current densities. Furthermore, it suppresses zinc deposition, or increases tin deposition at high current densities.
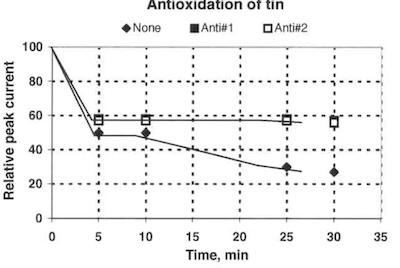 Fig. 5—Relative polarographic Sn(2+) peak signal over time after the addition of 100 μl H2O2.The relation between alloy composition and current density for the new proprietary process is shown in Fig. 4. The alloy composition is independent of the current density in the range investigated (0.1 to 4.0 A/dm2) and between 20 and 25 wt-% zinc. The alloy composition is measured using X-rays with a reproducibility of +/= 1 wt-%. A typical relation between alloy composition and current density is shown as the dash curve, and it is based on data from the literature.
Fig. 5—Relative polarographic Sn(2+) peak signal over time after the addition of 100 μl H2O2.The relation between alloy composition and current density for the new proprietary process is shown in Fig. 4. The alloy composition is independent of the current density in the range investigated (0.1 to 4.0 A/dm2) and between 20 and 25 wt-% zinc. The alloy composition is measured using X-rays with a reproducibility of +/= 1 wt-%. A typical relation between alloy composition and current density is shown as the dash curve, and it is based on data from the literature.
The oxidation of Sn(2+) to Sn(4+) is prevented by addition of a proprietary antioxidant. The oxidation is caused by dissolved air, which is the reason why air agitation cannot be applied to the process. Circulation of the electrolyte is created by filtration and eventually enhanced by mechanical movement of the parts.
A method based on polarography has been developed to evaluate the capability of the antioxidant to prevent oxidation of Sn(2+). The reduced polarographic current vs. time after the addition of 100ml H2O2 to 25 ml electrolyte is shown in Fig. 5. The reduction in polarographic Sn(2+) current is reduced compared to an electrolyte without the addition of an antioxidant (Anti #1 versus None in Fig. 5). A comparison to a commercially available antioxidant (Anti #2) is also shown in Fig. 5.
Table 2: Operational Range of New Tin/Zinc Process
| Tin (added as SnSO4) | 22–24 g/L |
| Zinc (added as ZnSO4) | 5-6 g/L |
| Complexant (proprietary) | 120–140 g/L |
| Brightener system (proprietary) | 6–10 ml/L |
| Antioxidant (proprietary) | 1–1.5 g/L |
| pH | 7.0–7.4 (adjusted with H2SO4 and NH4OH) |
| Temperature | 20–25°C |
| Current density | 0.5–4 A/dm2 |
| Agitation | None |
| Filtration | Yes |
| Anode | 75/25 tin/zinc anodes |
Summary
The laboratory data from testing alloy composition vs. corrosion resistance show optimal performance when the amount of alloying zinc is between 20 and 25 wt-%. The results are in agreement with the data in the literature.
A new process for the deposition of tin/ zinc alloys with optimal composition has been developed, and the amount of alloying zinc is independent of current density.
Dr. Jean Rasmussen is the founder of Jahl-Technology, Inc., 15816 Hwy. T, Maribel, WI 54227. Prior to starting his firm, he served as director of research & development at Pioneer Metal Finishing Corporation in Wisconsin. He has been a research chemist for surface treatment at Danfoss A/S and the Center for Advanced Electroplating at Denmark’s Technical University, Copenhagen, and has worked as a scientific assistant in environmental and nature conservation programs. He holds a BS in biology and an MS in electrochemistry from the University of Arhus, Denmark. He earned a PhD at Denmark’s Technical University.
Acknowledgement: The author is indebted to Chemtech Finishing Systems for assistance and support with this research project, and especially to Ed Budman from Chemtech Finishing Systems for his valuable support.
Operational Range: Based on results from development of the new proprietary tin/zinc process, the operational conditions are shown in Table 2.
Reference
1. Bech-Nielsen, G & Dorge, T.C., “Corrosion Measurements by Titration, CMT: A Rapid, Nondisturbing, Continuous Method for Measurements of Corrosion Rate,” Proc., AESF SUR/FIN Technical Conf. (1991).


































