Chrome plating, which has been around almost a century, is a technique of electroplating either a thin layer deposit (< 0.000015 inches) for decorative applications or thick layer deposit (>0.001 inches) for functional applications of chromium metal onto objects.
 Depending on the application, thin or thick layers provide corrosion resistance, ease of cleaning for a plated surface, or increasing the surface hardness and durability that helps to provide surface scuff resistance or improved wear performance. These ranges include many types of applications throughout many industrial sectors including automotive, plumbing, tools, and industrial equipment.
Depending on the application, thin or thick layers provide corrosion resistance, ease of cleaning for a plated surface, or increasing the surface hardness and durability that helps to provide surface scuff resistance or improved wear performance. These ranges include many types of applications throughout many industrial sectors including automotive, plumbing, tools, and industrial equipment.
Since 1924, decorative chrome plating has evolved to be aesthetically pleasing and durable for many applications including uses around the home, office, shopping centers. Traditionally chrome plating has been for enhancing the color of automotive wheels, steel parts or aluminum bumpers and various metal and plastic interior parts to make surfaces look great but also providing some resistance to scratches or resistance to other types of surface defects. Thickness ranges applied typical for many types of applications are from 2 micro-inches to 12 micro-inches (0.05 microns to 0.3 microns) and are normally plated over bright nickel electroplated layers to protect the integrity of the nickel surface while providing a good support layer for the chromium to shine!
Reducing Toxic Exposure
 As the basis of the decorative chrome plating systems, chromic acid, specifically hexavalent chromium is classified as toxic. There are regulations in all countries controlling the exposure of members of plating companies to chromic acid and especially resulting from the misting of CrVI emissions that form above the plating bath during the electroplating process. Historically, there are several proven preventative measures that have been routinely implemented to reduce this exposure but most widely utilized are mist/fume suppressant chemicals based on carbon-fluorine compounds.
As the basis of the decorative chrome plating systems, chromic acid, specifically hexavalent chromium is classified as toxic. There are regulations in all countries controlling the exposure of members of plating companies to chromic acid and especially resulting from the misting of CrVI emissions that form above the plating bath during the electroplating process. Historically, there are several proven preventative measures that have been routinely implemented to reduce this exposure but most widely utilized are mist/fume suppressant chemicals based on carbon-fluorine compounds.
Beginning in 1995, the U.S. Environmental Protection Agency recommended the use of Perfluorooctanesulfonic acid (better known as PFOS) as a fume-mist suppressant in the chromium electroplating process to protect workers from inhaling hazardous CrVI mists.
PFOS chemistry is highly resistant to chemical attack and so is ideally suited for use in harsh environments, especially these hot chromic acid decorative and hard chromium plating baths. Unfortunately, the extremely robust nature of PFOS means that it is not easily biodegraded or waste-treated and up until the recent past, it had been released into the environment where it can build up and accumulate. PFOS has been classified as a persistent, bioaccumulative and toxic (PBT) for some years now.
PFOS is an anthropogenic fluoro-surfactant and today recognized as a global pollutant. Outside of plating applications, PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and numerous stain repellents that we have used around our households for many decades.
The amounts of PFOS used in the plating industry have represented a tiny fraction of all commercial uses today. It’s estimated that the use of PFOS in the surface finishing industry represented less than one half of one percent of U.S. and the total global PFOS use. The surface finishing industry in 2012 voluntarily phased out the use of PFOS as a fume-mist suppressant for example utilized in chromic acid etching of plastics, and decorative chromium plating. The Surface Finishing Industry is the only entity to have proactively requested and received a ban on PFOS use in a — US-EPA regulation. The ban came into full effect in 2015.
The industry then adopted safer, EPA-approved, commercially available alternatives for fume-mist suppression, including both EPA-approved fluorinated and non-fluorinated based alternatives as fume suppressants.
Advanced Fluorinated Products
Today the industry relies on safer, more advanced fluorinated products for chromium fume suppression. Plating applicators are today working with their suppliers to find effective non-fluorinated alternatives as chromium fume suppressants that work similar to the legacy chemistries. The primary chromium fume suppressant today is still a fluorinated compound (referred to as 6:2 FTS, a polyfluorinated compound), which is rapidly eliminated from the body, has low toxicity, and is not bioaccumulative. However, this class of chemistry species today is becoming commonly referred to as PFAS, which, represents a large number of chemicals that have fluorine molecules bound to carbons in a chain. These are now under greater scrutiny for many reasons.
Currently, non-polymeric PFAS are the focus of regulatory concern; polymers are large, not bioavailable and not representing a high concern currently but still are under target. However, these non-polymer PFAS species can either have carbon atoms that are fully fluorinated and are referred to as per-fluorinated or have some carbon atoms also bound to hydrogen which are referred to as poly-fluorinated. There seems to be a lot of confusion still in our industry of surface finishing.
These per- and polyfluoroalkyl substances (PFAS) are a group of man-made chemicals characterized by a strong bond between fluorine and carbon. Because of this strong bond, PFAS provide resilience and durability. These properties are critical to the performance of hundreds of industrial applications and consumer products such as carpeting, apparel, upholstery, food paper wrappings, wire and cable coatings, and in the manufacturing of semiconductors.
The banned PFOS and perfluorooctanoicacid (PFOA) are examples of perfluorinated compounds that are also often referenced as “forever” chemicals are not used in surface finishing today.
The 6:2 FTS compound is an example of a polyfluorinated chemistry and less problematic but still, the negative content currently in the news media causes confusion and undue scrutiny to any use of this compound. The USA OSHA and the EPA continue to consider legislation that further justifies switching from hexavalent to trivalent chromium plating in order to avoid the use of any type of carbon-fluorine chemistry species.
From a health standpoint, trivalent chromium chemistry is intrinsically less toxic than hexavalent chromium. Because of the lower toxicity it is not regulated as strictly, it’s used in electroplating reduces applicator overhead costs and eliminates use of hazardous lead anode systems while not using any type of PFAS chemistry that will potentially be further restricted in the future.
Adopting to Meet Current Standards
Given all this confusion and continued scrutiny, why not adopt today a decorative plating technology that can meet all current demands today and into the future? Enter Trivalent Chromium plating technology. The TRISTAR Brand is the COVENTYA range of chemistries covering both chloride and sulfate electrolytes. There are a wide range of properties these platforms can provide but environmentally, these offer many advantages to the applicator for plating a chrome deposit.
Trivalent decorative chromium electroplating came into greater commercialization about 1975 after several years of successful use in Europe. There were many advantages realized, outside of the obvious environmental ones associated with chromic acid with removal of CrVI from waste effluents and plating mists. Eliminating the fussiness of deposit burning or chrome whitewash, parts could be removed from the plating tank and replated or could tolerate current interruptions. The systems provided increased throwing and covering power, eliminated the need for auxiliary anodes, had a micro-porous deposit structure thus eliminating the need for particle nickel layers, increased plating rate and did not require any anode or solution conditioning at startups or shut-downs. Corrosion performance was deemed pretty good and similar performance across many applications based on studies over the years including those realized by ASTM round robin testing and outdoor Kure Beach exposure evaluations. From a testing performance standpoint, trivalent chromium has found success already for some exterior applications in the trucking industry for the past 30 years. The recent past 2 – 3 year testing and reporting on status of actual field corrosion results by USCAR has also confirmed there is viability for switching to trivalent chromium for decorative applications. The sulfate/chloride processes have demonstrated a very long electrolyte life, process stability, ease of use, and excellent exterior corrosion performance.
Our chloride-based TRISTAR 300 trivalent chromium process has been industrialized and approved for many years in the automotive sector, where calcium chloride resistance (Russian mud) is considered a crucial parameter for some automotive specifications.
The newest, third-generation sulphate-based TRISTAR 330 AF trivalent chromium plating complies with stringent automotive specifications, and exceeds the PV1073 test in the Volkswagen TL528, which indicates very good resistance to calcium chloride.
Where Trivalent deposits have fallen short in comparison to hexavalent deposits was predominately deposit color and deposit “scuff resistance” or wear performance. People liked the shine and blue color aspect offered by hexavalent Chrome deposits so changing the attitude of OEM’s and others regarding the color acceptance differences has been a challenge and a reason why Coventya has developed a third generation system in TRISTAR 330 AF platform.
Indistinguishable From a Hexavalent Produced Deposit
Our TRISTAR 330 AF process enables the engineering community, applicators and the market to provide a deposit that is nearly indistinguishable from a hexavalent produced deposit. Measured in terms of the colorimetric scale *L,*a &*b, the resulting “L” value range of 83-85 is the same as that produced from a traditional decorative hexavalent deposit. The “b” value is less than zero on the scale which corresponds to a result touching the CrVI deposit value which has alluded Trivalent Chromium technology for decades. The TRISTAR 330 AF performance eliminates the dark and yellowish deposit aspect that has handicapped older generation trivalent processes now becomes history. The resulting color is very uniform across all part current densities, which results in excellent visual uniformity coming from production which is appreciated on assembled parts and components.
So today the only remaining concern is the question “How does the wear performance of a hexavalent decorative chrome plating deposit compare to a trivalent chrome deposit?”
A Coventya customer has realized many operational advantages of trivalent chemistry by electroplating seat belt buckle components with our TRISTAR 300 trivalent plating technology. The customer also produce parts with a proprietary hexavalent chrome (CrVI) plating system (fluoride catalyzed) for various OEM applications. In light of all the present focus on PFAS compounds in the environment, and how new pending regulations could change their operation, we did a study with them to evaluate how the wear resistance compares for our TRISTAR 300 deposit versus their hexavalent deposit as support to their customers for moving away from the CrVI targeted technology. This case study report will address these concerns.
To gauge how these deposits would compare, a Taber wear lab test (per ASTM B 504-90 update 2007) was chosen since that best mimics the performance of abrasion wear that can also be evaluated in a lab environment. In an automotive seat belt buckle system, the abrasion and resulting wear of plated surfaces can occur when two different bodies have opposing direction of movement as shown in the diagram. In the seat belt buckle systems, there is not significant contact loads, but the repetitive action of two opposing surfaces coming in contact can cause some type of wear to occur on the contact surfaces.
Abrasive wear as defined is the removal of material from a surface by a harder material impinging on or moving along the surface under load as shown in an exaggerated microscopic portrayal graphic:
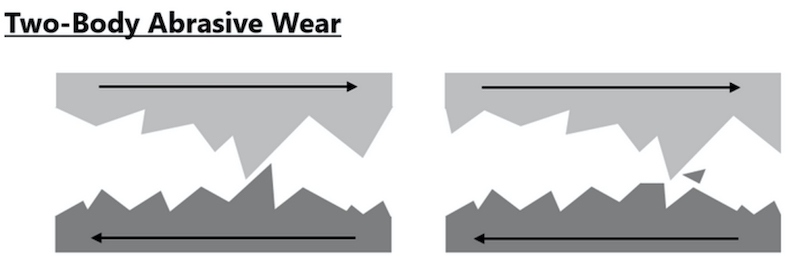
Wear is accelerated, as shown in the following diagram, when particles or other debris present in any application increases the contact area of the two surfaces during their opposing movements. This scenario is likely represented by the buckling insertion and unbuckling action of seat belt systems.
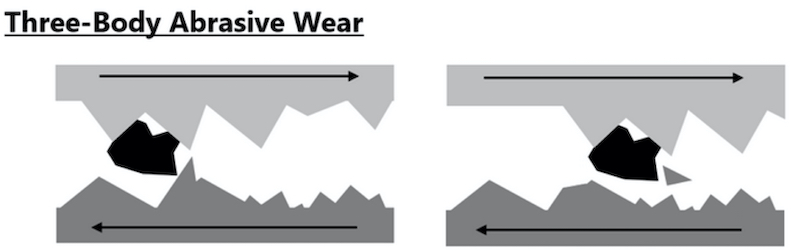
On two separate production occasions, three months apart, three (3) sets of steel Taber wear panels were processed through our TRISTAR 300 system at the customer. As reference, three (3) sets of hexavalent chrome plated taber wear panels were also processed at the customer serving as the basis for a CrIII to CrVI deposit comparison.
Processing Test Panels
All sets of panels were processed through the plating production line that includes proper surface preparation, activation and a nickel base layer (under layer) where both Cr(VI) and TRISTAR 300 Cr(III) deposits are produced for the lab evaluation. The target range of chrome deposit thickness for the majority of parts they process is 9 – 12 micro-inches. (0.23µ – 0.3µ). For reference, 0.1 mil = 0.0001 inches = 100 micro-inches.
Scientific Control Laboratories Inc., Chicago IL., with A2LA, Nadcap and NELAP accreditations, performed the Taber wear testing for all the samples. Two separate lab reports (19020496A & 19050086A) outline the procedures followed (per ASTM B 504-90 update 2007) and other details for the data generation and results. The testing was performed using a 500-gram load, CS 17 wheels and deposit integrity checked each 100 cycles for break-through to the nickel under layer. Surface temperatures of the panels during tested was monitored so any negative impact from heat generation during the testing could be eliminated. Taber wear results were reported as Average Wear Index loss of deposit (in micro-inches per cycle) to support a comparative analysis of the data to negate any differences with deposit thickness. In the analysis of the results, a lower number of micro-inches loss relates to improved wear performance and resulting in better resistance to abrasion wear.
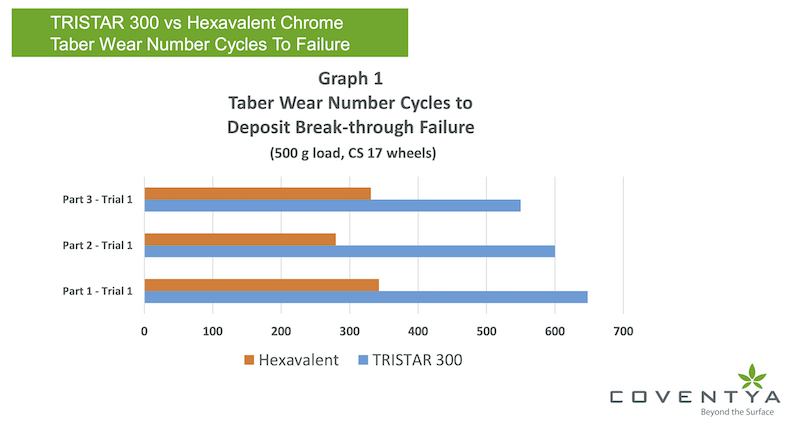
As shown in Graph 1, with a target range of deposit thickness from 9 – 12 micro-inches (0.23µ – 0.3µ) of either chrome plated deposit, the TRISTAR 300 deposit demonstrates the ability to reach about 2 times the number of taber wear cycle rotations (500 gram load, CS 17 wheels) versus the hexavalent deposit before the break-through failure of the deposit occurred. The break-through point of the failure to the plated layer represents visually seeing the steel base layer void of any chrome deposit. The number of cycles of rotation noted at that break-through or failure point with the TRISTAR deposit was an average 599 cycles while the hexavalent chromium deposit averaged 318 cycles.
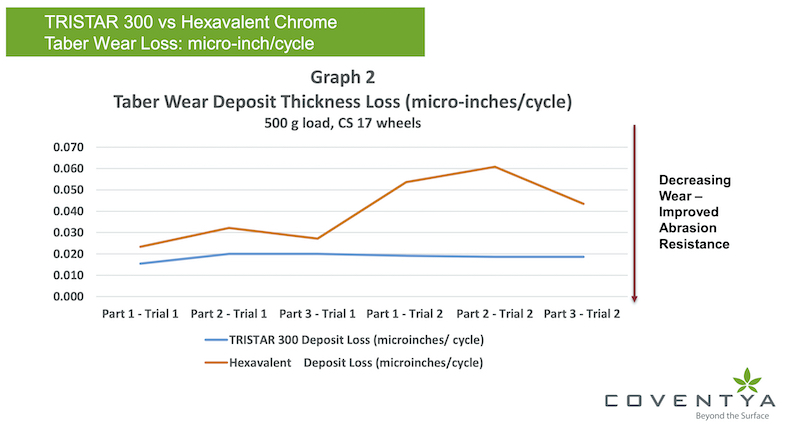
For Graph 2, we then look at the micro-inches thickness loss (deposit loss) per Taber Wear number of cycles to help correlate better the data and performance. As demonstrated, the TRISTAR 300 deposit over two separate trial evaluation periods has better wear performance to the hexavalent deposit. For trial 2, the thickness of the hexavalent chromium was lower than for trial 1 and that accounts most likely for the higher wear as shown in graph 2.
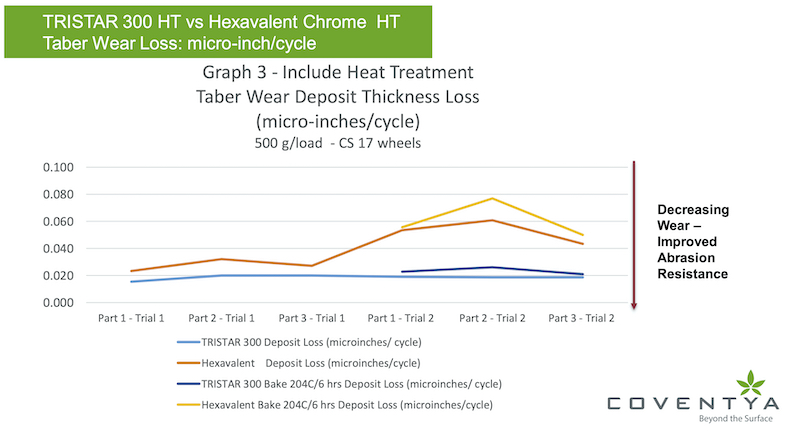
The series in graph 3 looks additionally at taber wear data for a post plating heat treatment process for both TRISTAR 300 and the hexavalent chrome deposit. For three of the six panels processed during the trial 2 series, these were post baked at 204°C for 6 hours. In each case comparing the heat treated to the non-heat treated samples, the loss of deposit expressed as micro-inches/cycle resulted in an increase in the wear (decreased wear resistance) by a resulting higher loss of thickness for this heat treatment process. Still, the TRISTAR 300 deposit demonstrates improved performance in wear and corresponding abrasion resistance to the hexavalent deposit.
Results/Conclusions:
The TRISTAR 300 system overall has higher thickness of deposit for similar plating time compared to the Cr(VI) system utilized in this evaluation which is also a benefit offering of Trivalent Technology.
Any doubts surrounding the performance of the TRISTAR 300 deposit should be mitigated in this case study focused on the ability of the deposit to provide wear (abrasion) resistance utilizing production test pieces, and lab apparatus for a comparative analysis.
In graph 1, comparing the 9 to 12 micro-inch thickness range, the TRISTAR 300 deposit provided 2 times the number of rotation cycles on the Taber Abrasion test machine using the 500 gram load and using CS-17 abrasion wheels compared to the hexavalent deposit.
Still, on a comparative basis of, looking at the thickness (deposit) micro-inches loss per taber wear cycle metric, both as deposited and after a bake process, the TRISTAR 300 deposit data demonstrates superior performance to the hexavalent deposit in this case study. It’s noted in both cases, the heat treatment bake process did degrade the wear performance of both deposits.
Utilizing the taber wear apparatus, in this case, represents a direct comparative test of one type deposit to another given similar conditions. It might or might not be exactly the wear performance expected in the real world we live today but on a comparative basis, this provides some strong signals. In the customer application of seat belt buckles and that requirement of repetitive insertions during use, the data indicates there should not be any detrimental impact of using trivalent TRISTAR 300 deposit for replacing a hexavalent deposit.
This case study does point to fact there are many advantages offered by looking at Trivalent chrome plating technology for replacing hexavalent, and in this specific case the TRISTAR 300 system and resulting deposit is a viable option.
In a worse case comparison, the TRISTAR 300 deposit demonstrated 33% less wear compared to the Cr(VI) deposit as averaging the data could indicate. Best case, there is a 60% less wear from the TRISTAR 300 deposit originating from the second trial series. In all fairness, that comparison could be impacted by the low range of Cr(VI) thickness from that series set but averaging both series of data indicates there is a 40 to 50% improvement in the deposit performance for TRISTAR 300 related to wear indices compared to the hexavalent deposit.
This testing and results of the case study provide a strong indicator that using a TRISTAR 300 (CrIII) deposit to replace a Cr(VI) deposit will not provide any insufficient wear or lower abrasion resistance compared to hexavalent deposits.
More importantly, with the Coventya Trivalent technology platforms, mitigating any need to use or require any type PFAS species or compound is an important step in the right direction toward providing a greener solution to plating decorative chrome deposits today and into the future.
If you have any questions or would like to discuss further, please contact Doug Lay (d.lay@coventya.com) or Brad Durkin (b.durkin@coventya.com).



































