Organic metal sounds like an oxymoron. Jumbo shrimp, government intelligence and walking dead come to mind, as well known examples that give us a chuckle on one hand but actually describe real things.
Organic metal is no different. The term describes a material that is carbon‐based (organic) but has properties of a metal, like conductivity, that you wouldn’t expect from an organic substance. It is a real thing and organic metals have special properties that make them useful for corrosion protection, oxidation prevention, final finishes and even as catalysts. They are environmentally friendly, requiring less energy to apply, reducing raw material usage and are generally non‐hazardous.
 Andy Lesko, PhDThere are many so‐called organic metals in existence: polypyrrole, polythiophene, polyphenyl sulfide, etc. In this article, the discussion will be solely devoted to polyanaline in a form, as manufactured by the MacDermid Enthone company. This patented form was developed by Dr. Bernhard Wessling and his company, Ormecon GmbH of Ammersbek, Germany, which was acquired by MacDermid Enthone in 2008.1
Andy Lesko, PhDThere are many so‐called organic metals in existence: polypyrrole, polythiophene, polyphenyl sulfide, etc. In this article, the discussion will be solely devoted to polyanaline in a form, as manufactured by the MacDermid Enthone company. This patented form was developed by Dr. Bernhard Wessling and his company, Ormecon GmbH of Ammersbek, Germany, which was acquired by MacDermid Enthone in 2008.1
This unique polymer is used in various products. Most notably it is used to passivate copper to provide a stable and solderable finish. The organic metal is also used in paints to improve corrosion protection for various metal substrates (originally marketed as CORRPASSIV). The polymer is available in a variety of solvents and concentrations for experimentation. This article is meant to introduce the uninitiated to this interesting material and possibly inspire others to give it another look.
Technical Features and Properties
An organic metal can also be called a conducting polymer or, more colloquially, a conducting plastic. Polyaniline, or PANI, as it is also known, has the form shown in Fig. 1.
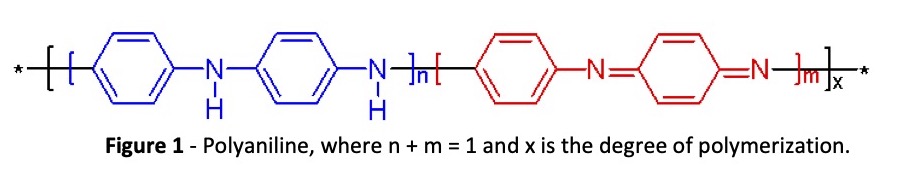
Polyaniline is a nitrogen hetero‐atom aromatic conducting polymer. As a bulk conducting plastic, it is generally not useful. However, if a solution of PANI existed, it might prove useful in various applications. This is exactly what was done by Ormecon GmbH. Through a unique process, PANI is dispersed in various solvents, including aqueous acid solutions, where one can now take advantage of its special properties and environmental benefits. This ability to create stable solvent dispersions of PANI results in a form known as the “organic metal.” In Table 1, a comparison is made of the conductivities of a few materials.
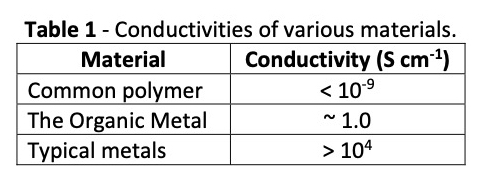
While the organic metal’s conductivity is not on the order of metals, it is relatively close and far above that of common polymers. Through the unique process developed at Ormecon GmbH, particles of PANI can be varied from approximately 10 to 100 nm in size, depending on the application. If these particles are now complexed with a small amount of silver, the result is the organic nanometal. The amount of silver represents less than 10% of the volume of a 50 nm deposit with silver present in the equivalent of a 4 nm‐thick layer. The complex is dispersed in an aqueous solution to utilize its unique properties.
The organic nanometal actually behaves as a new element. Measurements show that the nanometal has a standard reduction potential of 0.62 V. A portion of the electrochemical series is shown in Table 2.
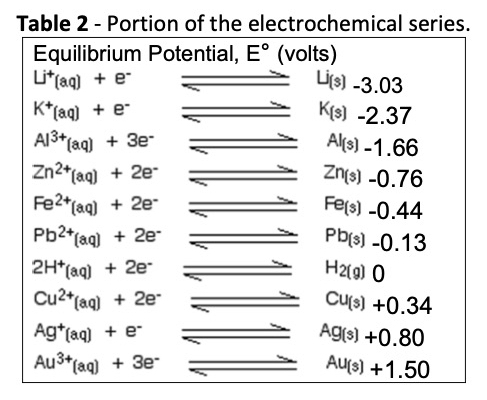
At 0.62 V, the nanometal falls between copper and silver. The result is that the nanometal should spontaneously deposit on elements above it in the series. This principle has been used to find application on copper, iron, and aluminum. Specifically, it passivates copper and protects it against oxidation until it can be soldered or further processed. This passivation is demonstrated by the Kelvin potentials in Fig. 2.
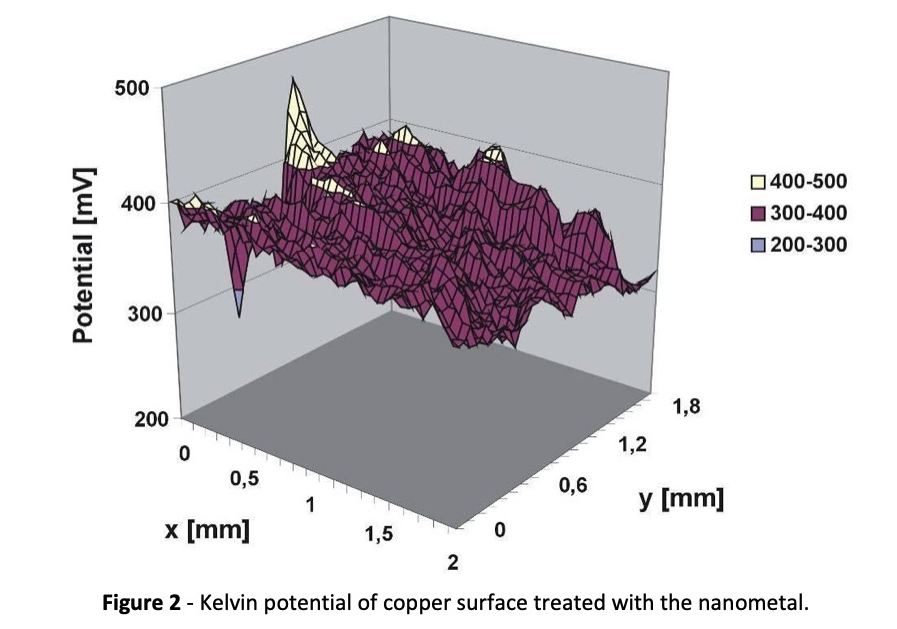
Kelvin potentials measure the work function of various materials in close proximity to each other. The work function is the property of a material to release electrons to another material, or the ability to oxidize the surface. Table 3 shows the relative position of the nanometal among other surface conditions.
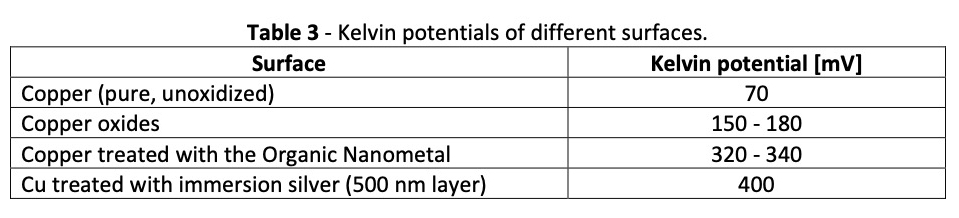
Copper, with a Kelvin potential of 70 mV, is much easier to oxidize than a copper surface treated with the nanometal. A copper surface coated with pure silver is only slightly less sensitive to oxidation than one coated with a nanometal ten times thinner.
A scanning electron microscope image (SEM) of the nanometal on copper is revealing. The passivation appears to be achieved, not by a barrier coating, but by a net‐like structure as shown in Fig. 3.
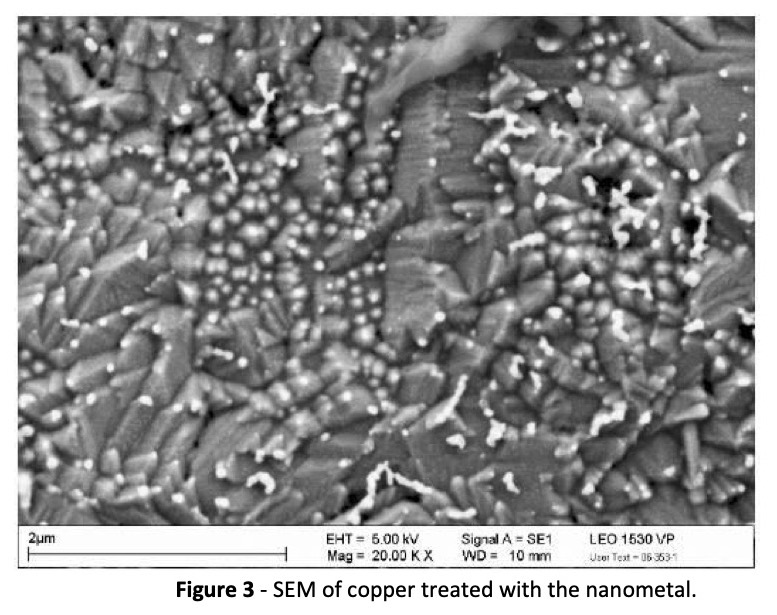
It is believed that the passivation comes from the nanometal complex preferentially depositing on grain boundaries where copper oxidation begins and thus raising the Kelvin potential.
The nanometal features and properties have been well studied and there are numerous reports showing its unique properties that are beyond the scope of this article.2
Application
The full extent of the application of conducting polymers is probably not known. Some known creative applications are: stimulus‐responsive biomaterials, fuel cells, electroluminescent displays, anti‐static coatings, and, of course, corrosion protection. Here we’ll discuss two applications related to surface treatment: corrosion protecting paints and surface treatment of copper.
Corrpassiv is a product developed by Ormecon GmbH. It incorporates the organic metal in various paints and lacquers and passivates the surface to which it is applied. When contact is established between the organic metal and the metal to be protected, a dual protective mechanism takes effect. Industrial coatings, and seawater and offshore applications are the main areas where Corrpassiv has seen use. Figure 4 below shows how it is used in a primer coating to provide exceptional corrosion protection.
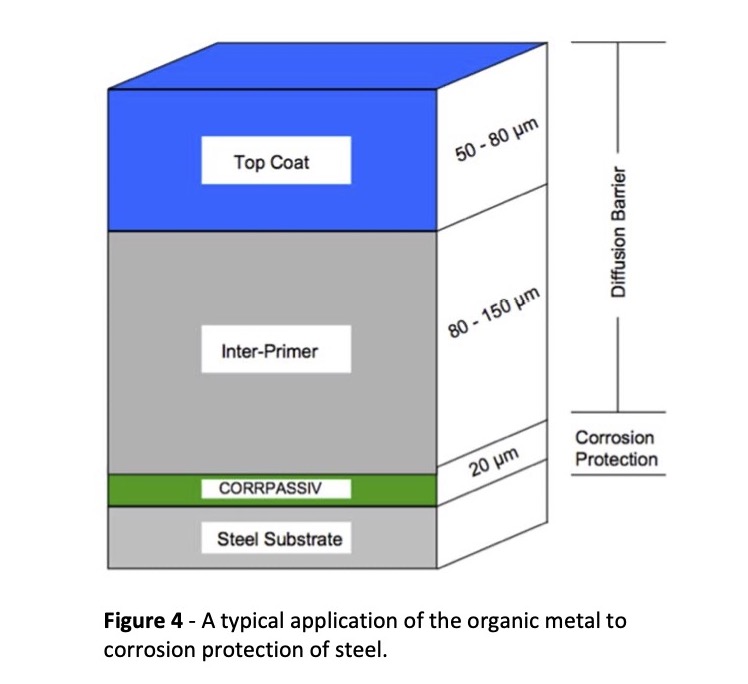
Numerous industrial structures from bridges to pipe coatings to ships to power plants have used the Corrpassiv process to enhance corrosion protection.
While corrosion protection through paints and lacquers has shown success, MacDermid Enthone currently and most actively applies the organic metal to protecting copper surfaces of printed circuit boards (PCBs). Two products, the CSN immersion tin process and the Entek OM process, serve to provide alternatives to traditional final finishers for PCBs prior to soldering.
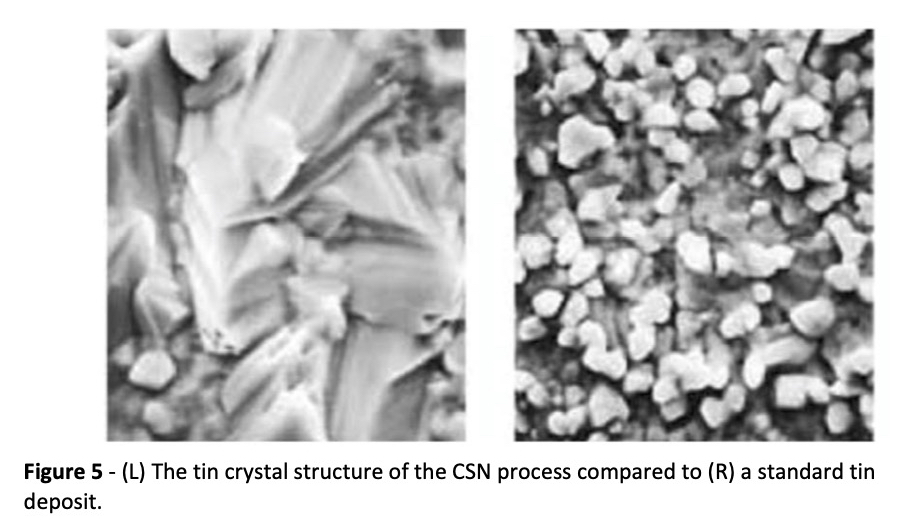
CSN immersion tin uses the organic metal as a pretreatment for copper prior to coating with tin. The pretreatment has the benefit of catalyzing the deposition of tin and helps reduce the copper‐tin diffusion process which can lead to tin whiskers; an undesirable defect. The organic metal accomplishes this by promoting larger than normal tin grain structures. A longer shelf life for PCBs is also enjoyed by using this process. Figure 5 shows the difference in grain structure.
Another application for copper protection is the use of MacDermid Enthone’s Entek®OM.3 This is a stand‐alone finish that makes use of the passivating properties of the nanometal to provide a solderable finish for PCBs without the use of a metallic coating. As discussed earlier, the nanometal raises the Kelvin potential of the surface making it more difficult to oxidize. Figure 6 shows how treated copper withstands oxidation after aging.
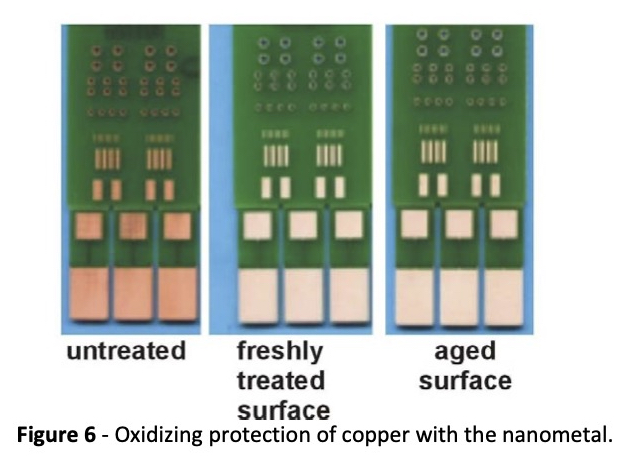
Along with fulfilling a need to preserve copper surfaces, Entek®OM offers significant environmental advantages.
Environmental Impact
The fact that metal does not need to be mined from the earth to produce and use the organic metal suggests that it is more environmentally‐friendly than metallic surface finishes. Even without taking mining resources into account, the organic metal process and application has less environmental impact. Here, we discuss it in terms of its use as a PCB finish.
If we consider only the deposition of metal on to the PCB and consider energy, water and metal demands, the organic metal fares much better. Without going into the details of the various PCB surface finishes,4 all have higher energy use than the Entek®OM process. In particular, ENIG, with its high water and energy consumption, is the worst offender, yet the most popular finish with an estimated use of around 60% of all PCB final finishes.
By comparing the necessary energy requirements for application of final finishes, we estimate that Entek®OM has a 66% less energy demand than an average high energy demand process like immersion tin. Compared to ENIG, Entek®OM needs only 10% of that energy demand. For a typically low energy demand finish like OSP, Entek®OM still yields about a 50% energy savings. Figure 7 shows this relative comparison.
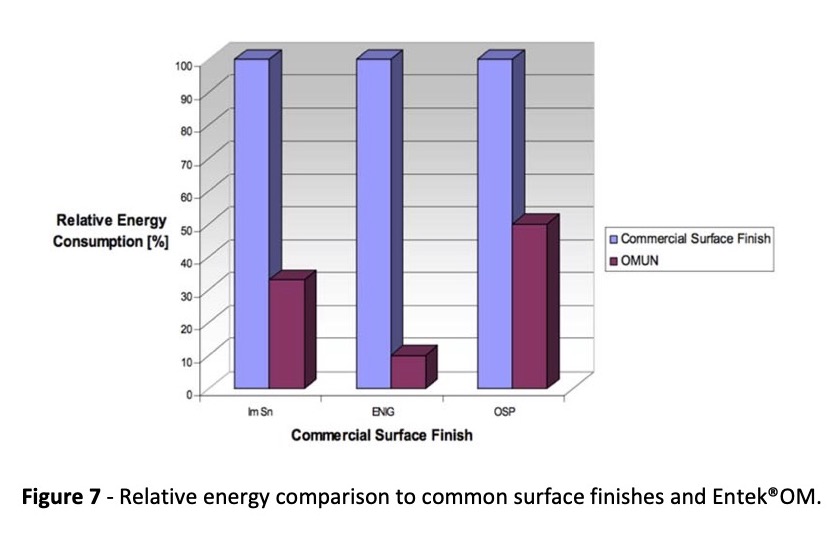
Based on surface finish application only, the Entek®OM process has the possibility of reducing water demand by 70%, energy demand by 50‐95%, and metal demand by 99%.
This discussion of environmental impact is meant to be a cursory look at the environmental impact of a non‐metal technology to replace traditional metallic finishes, but more work needs to be done.
Conclusion
The purpose of the discussion here is to raise awareness of alternative technologies for process improvement and introduce a unique material, the conducting polymer, or in our case the organic metal. This surface treatment can be applied to prevent corrosion and provide other useful features. The use of conducting polymers in the metal finishing sector is largely unknown. We at MacDermid Enthone have taken steps to advance and promote this unique material as an alternative to traditional finishes. Since most modern developments in industrial chemicals are driven by regulation, it might be time to take a another look at conducting polymers as a pathway to meeting regulatory requirements while still providing necessary metal finishing properties.
About the Author
 Andy Lesko, PhDDr. Andy Lesko is a graduate of the University of Michigan with a Ph.D. in chemistry and a Six Sigma Master Black Belt certified engineer. He has worked in manufacturing for over 20 years building printed circuit boards, processing specialty chemicals and currently collaborating as a Six Sigma Program Manager with MacDermid‐Enthone Industrial Solutions. He services the metal finishing industry, helping customers solve problems, optimize processes, and run Six Sigma projects. He also works as a process control expert deploying the MacDermid‐Enthone M-Logic software system. Andy lives near Portland, Oregon, supporting MacDermid‐Enthone customers within the whole American continent.
Andy Lesko, PhDDr. Andy Lesko is a graduate of the University of Michigan with a Ph.D. in chemistry and a Six Sigma Master Black Belt certified engineer. He has worked in manufacturing for over 20 years building printed circuit boards, processing specialty chemicals and currently collaborating as a Six Sigma Program Manager with MacDermid‐Enthone Industrial Solutions. He services the metal finishing industry, helping customers solve problems, optimize processes, and run Six Sigma projects. He also works as a process control expert deploying the MacDermid‐Enthone M-Logic software system. Andy lives near Portland, Oregon, supporting MacDermid‐Enthone customers within the whole American continent.
References
- B. Wessling, Handbook of Conducting Polymers (T. Skotheim, R. L. Elsenbaumer, and J. R. Reynolds, eds.), Dekker, New York (1998).
- B. Wessling, Polymers 2010, 2 (4), 786‐798; doi:10.3390/polym2040786.; B. Wessling and J. Posdorfer, “Corrosion Prevention with an Organic Metal (Polyaniline): Corrosion Test Results,” Electrochimica Acta 44, 2139‐2147 (1999).; B. Wessling, http://pubs.acs.org/subscribe/archive/ci/31/i01/html/01wess.html; https://www.researchgate.net/publication/256039692_Dispersion_as_the_Key_to_Processing_Conductive_Poly mers.
- J. Kenny, et al., https://www.smta.org/knowledge/proceedings_abstract.cfm?PROC_ID=3358
- Hot air solder level (HASL), electroless nickel immersion gold (ENIG), immersion tin, immersion silver and organic solderability preservative (OSP).



































