The history of electroplating is a curious mixture of mistakes, observations, serendipity and experimental development, enmeshed and entwined with the discovery of electricity in the late 18th century.
 Bill NebioloJoin me as I review the fascinating history of our industry and its metamorphosis during the last 238-years. I will also provide a brief history of our flagship society, the NASF.
Bill NebioloJoin me as I review the fascinating history of our industry and its metamorphosis during the last 238-years. I will also provide a brief history of our flagship society, the NASF.
Have you ever wondered who was the first individual to electroplate something? Have you ever heard the term Galvanic action and pondered its origin? Are you aware that the French Emperor Napoleon played a role in our industry? Who discovered the first nickel brightener? These are just a few of the fascinating tidbits that will be shared in this fascinating, light-hearted and enjoyable presentation.
The History of Electroplating
 Luigi GalvaniLuigi Galvani, was born in Bologna, Italy on September 9, 1737.16 As a young man Galvani was interested in the field of theology. But encouraged by his parents to study the sciences, he instead enrolled at the University of Bologna in 1755 and received his Baccalaureate in Science in 1759.16
Luigi GalvaniLuigi Galvani, was born in Bologna, Italy on September 9, 1737.16 As a young man Galvani was interested in the field of theology. But encouraged by his parents to study the sciences, he instead enrolled at the University of Bologna in 1755 and received his Baccalaureate in Science in 1759.16
Following his father’s preference for medicine, he then pursued; as a graduate student the field of physiology and was awarded a medical degree in 1762. In 1781, the new and exciting science of the time was the recent discovery and study of electricity. In 1774, Galvani read a paper published by three authors; Leopoldo Caldani, Felice Fontana and Tommaso Laghi, who found that muscles in frogs, could be activated by the application of electrical stimulation.16
Curious of the claims in this paper, Galvani began his own series of experiments on severed frog legs, seeking to duplicate the findings of his peers and understand the phenomenon.
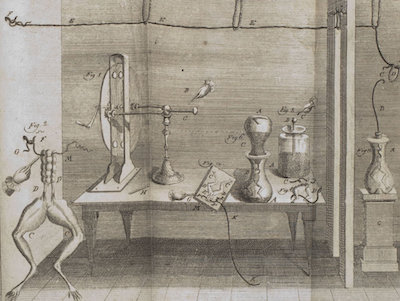 A 1781 Drawing from Galvani’s notebook.This image; from Galvani’s laboratory notebook, shows that from 1774 to 1781 he used a hand-cranked static electricity generator and Leyden jar in his experiments. Touching the sciatic nerve in a severed frog’s leg; with a scalpel that had picked-up a charge from the Leyden jar, Galvani noted that the frog’s legs would indeed contract.16
A 1781 Drawing from Galvani’s notebook.This image; from Galvani’s laboratory notebook, shows that from 1774 to 1781 he used a hand-cranked static electricity generator and Leyden jar in his experiments. Touching the sciatic nerve in a severed frog’s leg; with a scalpel that had picked-up a charge from the Leyden jar, Galvani noted that the frog’s legs would indeed contract.16
Continuing his experiments, Galvani made an interesting discovery. After pinning a frog’s leg to his lab table with a wrought iron nail; and before he connected the copper probe to the Leyden jar, he accidentally touched the frog’s sciatic nerve. To his surprise the frog’s leg contracted. Galvani; however was perplexed. How could this be since he hadn’t yet electrified his copper probe? He repeated the procedure with the same result and eventually concluded erroneously that the frog itself was its own source of electricity.2, 3, 5, 16
Italian physician; Luigi Galvani, had discovered what he called “animal electricity.”5 He claimed that an electrical fluid within the frog’s body was carried to the muscles by its nerves.5 After additional experimentation, Galvani determined that by connecting any two different metal probes in series with wires inserted into a frog’s leg, he could stimulate the leg to contract.5, 16
Alessandro Volta
 Alessandro VoltaIn 1791, Galvani published the results of his experimentation and erroneous conclusion that the frog itself was the source of its own electricity.5,16 At the University in Padua, Italy, Italian physicist; Alessandro Volta, read Galvani’s paper and began to expand upon Galvani’s animal electricity work. Volta eventually replaced the frog’s leg with a brine-soaked piece of paper. In doing so, Volta was able to detect the same flow of electricity that Galvani had detected, as long as the two probes that he used to connect to the brine-soaked paper in series, were made from dissimilar metals.2, 3, 5, 16
Alessandro VoltaIn 1791, Galvani published the results of his experimentation and erroneous conclusion that the frog itself was the source of its own electricity.5,16 At the University in Padua, Italy, Italian physicist; Alessandro Volta, read Galvani’s paper and began to expand upon Galvani’s animal electricity work. Volta eventually replaced the frog’s leg with a brine-soaked piece of paper. In doing so, Volta was able to detect the same flow of electricity that Galvani had detected, as long as the two probes that he used to connect to the brine-soaked paper in series, were made from dissimilar metals.2, 3, 5, 16
Volta also determined that if the two probes were of identical metal then there would be no flow of electricity. Volta had actually developed the first wet cell battery or as it was known at the time, a Voltaic Pile.3
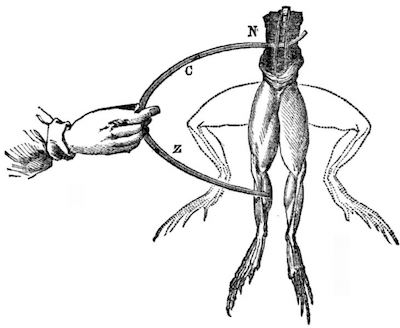 Galvani notebook image of contracting leg.It was Volta who realized that the frog’s leg in Galvani’s experiments; served as both the electrolyte; or carrier of the electrical current, and as the detector of the current, when the leg contracted.3
Galvani notebook image of contracting leg.It was Volta who realized that the frog’s leg in Galvani’s experiments; served as both the electrolyte; or carrier of the electrical current, and as the detector of the current, when the leg contracted.3
The Voltaic Pile generates electrical current due to the difference in electrochemical potential between two different elements. This phenomenon is known as EMF; or, electromotive force. The further the separation between two different elements the larger the electromotive force that pair can generate when connected in series.2, 3 By experimenting with different metals, Volta was able to generate a chart of the possible voltage potentials when anodes and cathodes of different metals are combined in a voltaic pile or a battery.2, 3
Table No. 1; the Electromotive Series16
Metal: Voltage Potential
- Lithium: -3.04 volts
- Calcium: -2.87 volts
- Magnesium: -2.37 volts
- Aluminum: -1.66 volts
- Zinc: -0.76 volts
- Chromium: -0.74 volts
- Iron: -0.44 volts
- Hydrogen: 0.00 volts
- Copper: +0.34 volts
- Silver: +0.80 volts
- Gold: +1.68 volts
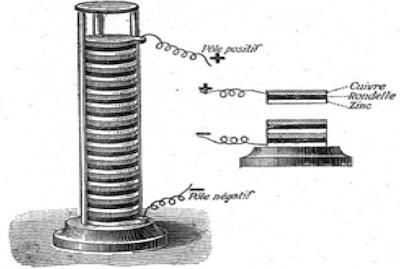 The Voltaic pile of 1800. This first dependable, continuous source of D.C. electrical current.16Volta’s first, practical, voltaic pile consisted of alternating layers of zinc and copper separated by brine soaked cardboard to which a small amount of dilute sulfuric acid was added.2, 3, 5, 16
The Voltaic pile of 1800. This first dependable, continuous source of D.C. electrical current.16Volta’s first, practical, voltaic pile consisted of alternating layers of zinc and copper separated by brine soaked cardboard to which a small amount of dilute sulfuric acid was added.2, 3, 5, 16
As a member of The French Institute of Science; the most prestigious scientific society of its day, Alessandra Volta travelled to France in 1801, where he demonstrated the Voltaic Pile before scientific colleagues and members of the Institute at the court of French Emperor Napoleon Bonaparte.5, 16
Volta’s Law or the Law of Electromotive Force states that the amount of electricity a battery will generate is proportional to the difference between the electrochemical potentials of the two metals used as its electrodes.16 To this day, electrical voltage is named in Volta’s honor for his discoveries and contributions. Likewise, the principle of Galvanic Action; wherein oxidation occurs at the anode and reduction occurs at the cathode, is named to honor Luigi Galvani.16
Luigi Brunatelli; the First Electroplater
 Luigi Brugnatelli16A colleague of Alessandro Volta, also a member of the French Institute of Science, Italian chemist, Luigi Brugnatelli, traveled to Paris in 1801 where he witnessed Volta’s demonstration of the voltaic pile.5 Intrigued with the device and proposing chemical experiments to be carried out with electricity, Brugnatelli was chartered; under the auspices of the French Institute of Science and funded by the French government of Emperor Napoleon Bonaparte. He experimented with the voltaic pile at his chemical laboratory, upon returning to Italy.5 Four years later in 1805; Luigi Brugnatelli, became the first person to electroplate another item.5
Luigi Brugnatelli16A colleague of Alessandro Volta, also a member of the French Institute of Science, Italian chemist, Luigi Brugnatelli, traveled to Paris in 1801 where he witnessed Volta’s demonstration of the voltaic pile.5 Intrigued with the device and proposing chemical experiments to be carried out with electricity, Brugnatelli was chartered; under the auspices of the French Institute of Science and funded by the French government of Emperor Napoleon Bonaparte. He experimented with the voltaic pile at his chemical laboratory, upon returning to Italy.5 Four years later in 1805; Luigi Brugnatelli, became the first person to electroplate another item.5
Brugnatelli electroplated gold onto two silver medallions, by connecting them in series to a gold anode.5, 13 All of the items were immersed in a brine solution and connected to a voltaic pile.5, 13 The work; done under the auspices of the French Academy of Science, was ordered by Emperor Napoleon Bonaparte to remain a secret. This proclamation prohibited Brugnatelli from publishing of his scientific breakthrough; essentially, the development of electroplating. Thus suppressed, his work remained unknown for several decades until, he was allowed to publish a treatise on his electroplating developments in The Belgian Journal of Physics and Chemistry.13
The Contributions of Michael Faraday
 Michael Faraday16The suppression of Brugnatelli’s developments prevented the discovery from being known in scientific literature and as a result the science of electroplating was reinvented in England. To that end, investigatory work on electricity; continued in London, during the 1820s and 1830s at the laboratory of famed English scientist; Sir Humphry Davy.13 A protégé in Davy’s laboratory was chemist; Michael Faraday.11
Michael Faraday16The suppression of Brugnatelli’s developments prevented the discovery from being known in scientific literature and as a result the science of electroplating was reinvented in England. To that end, investigatory work on electricity; continued in London, during the 1820s and 1830s at the laboratory of famed English scientist; Sir Humphry Davy.13 A protégé in Davy’s laboratory was chemist; Michael Faraday.11
In 1833, Faraday made some interesting observations during some of his experiments.3, 4, 5, 13 Those observations led to the establishment of the laws of modern electroplating. To this day the law’s still bear his name and establish electroplating operations on firm scientific principles.11 They are the basis for every electrolytic electroplating operation in use to this very day.
Faraday’s First Law of Electrodeposition: The amount of chemical change produced by an electric current; (i.e. the amount of metal deposited cathodically or dissolved anodically,) is proportional to the quantity of electricity passed through the plating bath.2, 3, 11
Simply stated; Faraday’s first law says, that if you apply more current to the bath, you’ll deposit more metal.16
Faraday’s Second Law of Electrodeposition: The weight of different metals deposited or dissolved by the same quantity of electricity is proportional to their chemical equivalent weights.2, 3, 11
Simply stated; Faraday’s second law says, if the current remains the same different metals deposit to different depths during the same period of time dependent upon their equivalent weight or valency.16 So for the non-chemists among us the question is, “What is a metal’s equivalent weight?”
The equivalent weight of a metal is simply its atomic weight; as found on the periodic chart, divided by its valence.11 For example; nickel has the atomic weight of 58.70 grams and a normal valence of 2. Therefore its equivalent weight is 58.70 grams divided by 2, or 29.35 grams.11 Silver has an atomic weight of 108.87 grams and a valence of one, therefore, it has an equivalent weight of 108.87 grams divided by one, or 108.87 grams.11
By combining Faraday’s first and second laws we are able to create the Metal Deposition Formula.3, 11
Metal Deposition Formula3, 11
M = (I)(t)(E)(1/F) where:
- M = Metal weight in grams deposited
- I = current in amps
- t = time in hours
- E = the metal’s equivalent weight
- 1/F = amp hour equivalent
But what is 1/F the amp hour equivalent? Faraday noted that a current of 1 amp for 1 second deposits 0.001118 grams of silver. Silver has an equivalent wt. of 107.87 grams. By dividing silver’s equivalent weight of 107.78 grams by 0.001118 g of silver per second Faraday determined that to deposit 107.78 grams of silver would require 96,494 amp seconds.2, 3, 11 This has since been round up to 96,500 amp seconds for convenience. In electroplating, amp seconds are referred to as coulombs and the standard of 96,500 coulombs is called 1 Faraday in honor of the scientist.11
John Wright and the Elkingtons
 George Elkington1635-years after Italian chemist; Luigi Brugnatelli first electroplated two silver medallions with gold, Birmingham, England based tableware and jewelry manufacturer; John Wright discovered that potassium cyanide was a suitable electrolyte for gold and silver electroplating.4, 5, 13 Other inventors in Birmingham England were also tinkering with the technology at the time4, 5, 13 but it was Wright who first published and was awarded a patent for the technique. A report detailing Wright’s patent was published in a 1840 edition of The Birmingham Jewelry Quarterly.4, 5, 14 With that publication the spread of the knowledge related to the science of electroplating was begun. Wright is sometimes erroneously crediting as the developer of electroplating; a false claim as the credit goes to Brugnatelli for his work in 1805, Wright was simply the first to publish an account of the technology as Brugnatelli’s work was suppressed by French Emperor Napoleon Bonaparte.16
George Elkington1635-years after Italian chemist; Luigi Brugnatelli first electroplated two silver medallions with gold, Birmingham, England based tableware and jewelry manufacturer; John Wright discovered that potassium cyanide was a suitable electrolyte for gold and silver electroplating.4, 5, 13 Other inventors in Birmingham England were also tinkering with the technology at the time4, 5, 13 but it was Wright who first published and was awarded a patent for the technique. A report detailing Wright’s patent was published in a 1840 edition of The Birmingham Jewelry Quarterly.4, 5, 14 With that publication the spread of the knowledge related to the science of electroplating was begun. Wright is sometimes erroneously crediting as the developer of electroplating; a false claim as the credit goes to Brugnatelli for his work in 1805, Wright was simply the first to publish an account of the technology as Brugnatelli’s work was suppressed by French Emperor Napoleon Bonaparte.16
 Ad for Elkington Electro Plate.10At the time; as it is often done today, electroplating was simply a means to reduce cost. Items could be made inexpensively from a basis metal then decoratively electroplated so as to appear that they were manufactured in whole, from the precious metal.13, 14
Ad for Elkington Electro Plate.10At the time; as it is often done today, electroplating was simply a means to reduce cost. Items could be made inexpensively from a basis metal then decoratively electroplated so as to appear that they were manufactured in whole, from the precious metal.13, 14
Electroplating patent owner; John Wright, was a business partner with Elkington brothers Henry and George at the trio’s Birmingham-based tableware and jewelry enterprise. Recognizing the value of the patent, the Elkington’s purchased the rights to the gold and silver potassium cyanide electroplating technique from their partner John Wright and bought-out his interest in the tableware and jewelry business.12
Now, as owners of the patent, the Elkington brothers, opened the world’s first electroplating job shop. Renaming their business the Elkington Plating Works they also continued to manufacture their fine-line of tableware and jewelry and additionally accepted gold and silver electroplating job shop work.
 Lithograph of the Elkington Plating Room.5Holding a virtual monopoly with the gold and silver electroplating patent rights the Elkington Plating Works prospered significantly from the original Wright patent and enjoyed the rights and privileges the patent afforded to them for the years of its exclusivity. Thus with a head start over others in the field of commercial decorative electroplating, the Elkington Plating Works meteoric growth peaked with a workforce of some 1,000 employees by 1890.12 Its original building still stands today in Birmingham.10, 16
Lithograph of the Elkington Plating Room.5Holding a virtual monopoly with the gold and silver electroplating patent rights the Elkington Plating Works prospered significantly from the original Wright patent and enjoyed the rights and privileges the patent afforded to them for the years of its exclusivity. Thus with a head start over others in the field of commercial decorative electroplating, the Elkington Plating Works meteoric growth peaked with a workforce of some 1,000 employees by 1890.12 Its original building still stands today in Birmingham.10, 16
The Woolrich Generator
Since the voltaic pile was essentially; a battery, its disadvantage would be the loss of D.C. current and an interruption to the plating cycle when its anodic material depleted. By 1844, the voltaic pile was replaced by a more reliable D.C. power source, the Woolrich Generator.10
 Woolrich Generator Birmingham Science Museum, Birmingham, England.10In 1842, chemist; John Stephen Woolrich, patented his idea for a D.C. electrical generator. Two years later in February, 1844 his newly created company; The Magneto Works Co., sold its first D.C. generator to the Birmingham tableware company Thomas Prime & Sons, for the silver electroplating of tableware and hollowware.10
Woolrich Generator Birmingham Science Museum, Birmingham, England.10In 1842, chemist; John Stephen Woolrich, patented his idea for a D.C. electrical generator. Two years later in February, 1844 his newly created company; The Magneto Works Co., sold its first D.C. generator to the Birmingham tableware company Thomas Prime & Sons, for the silver electroplating of tableware and hollowware.10
The Woolrich generator was a magneto-type generator using permanent magnets to create the magnetic field in which the windings rotate.10 In contrast, most generators use electromagnets in which a current flows through a coil to create the magnet field. Electromagnets can produce stronger fields; and importantly, the field can be varied to adjust the voltage generated.10 Electroplating requires a direct current, so the Woolrich generator was fitted with an early commutator.10
Nickel as a Replacement for Silver Plate; Dr. R. Bottger & the First Nickel Bath
About this time, 1840–1844, an interest developed in the replacement of silver electroplate with nickel electroplate. The primary advantage was that nickel was less expensive to purchase than silver. Then, as is now, overhead cost savings were an important part of any business’ profitability.
In 1843, German physician turned electroplating scientist; Dr. R. Bottger, developed the first practical formulation for the electroplating of nickel.2, 5 Bottger’s aqueous solution of nickel and ammonium sulfate remained the basic nickel electroplating formulation for the next 70 years.5
William H. Remington Anode Baskets
In Boston, MA in 1866; businessman; William H. Remington additionally realized a second advantage to nickel electroplating vs. silver electroplating that being, a nickel electroplated coating could be buffed to a mirror reflective condition.16 Due to its anti-corrosive properties the nickel coating would remain bright, reflective and lustrous. Whereas silver and silver electroplate have to be routinely buffed as they continuously tarnish.16
That year, Remington established the William H. Remington Co. for the decorative electroplating of nickel and silver.5 Remington contributed to the advancement of the science, with his 1868 invention of the anode basket.5 Remington received U.S. Patent No. 82,877 in Oct. 1868 for the device.16
Dr. Isaac Adams; Job Shop Nickel
 Dr. Isaac Adams Jr.5Born in Boston, MA in 1836, Isaac Adams Jr. attended Bowdoin College in Brunswick, ME from which he received his Bachelors Degree in 1858.5 He then attended Harvard Medical School receiving his MD in 1862 which he followed with two additional years of study at the Êcole de Médicine in France.5 Returning to Boston he opened his medical practice; which he abandoned for reasons unknown, just two-years later in 1866.
Dr. Isaac Adams Jr.5Born in Boston, MA in 1836, Isaac Adams Jr. attended Bowdoin College in Brunswick, ME from which he received his Bachelors Degree in 1858.5 He then attended Harvard Medical School receiving his MD in 1862 which he followed with two additional years of study at the Êcole de Médicine in France.5 Returning to Boston he opened his medical practice; which he abandoned for reasons unknown, just two-years later in 1866.
He then opened a chemistry laboratory in Boston and delved into the topic of the day for electroplaters; the advancement of nickel electroplating.5 Employing the R. Bottger nickel plating bath of 1843, in just three years of experimentation, Dr. Adams developed an improved nickel electroplating procedure.5, 12
Dr. Adams received U.S. Patent No. 98,157 on August 8, 1869 for Improvements to the Electro-Deposition of Nickel.5, 12 In his improved bath Adams substituted the double salt of nickel ammonium sulfate for Bottger’s use of the single salt of ammonium sulfate. Adams also enhanced bath performance by maintaining the bath pH value at 45, 14 with the periodic addition of sulfuric acid.
 Notes from Dr. Adams’ laboratory notebook from July 26, 1866.5With a bath pH value of 4, Adams was able to temper the generation of ammonium hydroxide, the result of oxygen generation at the anode. He found that the higher pH value lowered cathode efficiency and embrittled the nickel plate.5 With patent in hand Adams purchased the William H. Remington Co. in Boston in 1869 and renamed it the Boston Nickel Plating Co.5, 16 There he employed his new nickel plating bath and continued to commercially electroplate silver on a job shop basis.
Notes from Dr. Adams’ laboratory notebook from July 26, 1866.5With a bath pH value of 4, Adams was able to temper the generation of ammonium hydroxide, the result of oxygen generation at the anode. He found that the higher pH value lowered cathode efficiency and embrittled the nickel plate.5 With patent in hand Adams purchased the William H. Remington Co. in Boston in 1869 and renamed it the Boston Nickel Plating Co.5, 16 There he employed his new nickel plating bath and continued to commercially electroplate silver on a job shop basis.
Three years later; in 1872, he opened the first, commercial, exclusively nickel-plating job shop in the U.S. That company; which he named the Adams Plating & Manufacturing Co., was located in the town of South Windham, CT.5 Adams additionally adopted Remington’s use of anode baskets to hold the bath’s anodes.5
Dr. Isaac Adams Jr. is credited with being the father of nickel plating in the United States.5, 14
Dr. Edward Weston and Boric Acid
 Dr. Edward WestonBorn in England in 1850, Dr. Edward Weston; also trained as a physician, but turned awy his medical training. Landing in New York City in 1870, he too soon began experimenting with nickel electroplating improvements.5, 13
Dr. Edward WestonBorn in England in 1850, Dr. Edward Weston; also trained as a physician, but turned awy his medical training. Landing in New York City in 1870, he too soon began experimenting with nickel electroplating improvements.5, 13
Unable to determine an end-around to bypass Adams’ patent, Weston was nonetheless, the first person to introduce boric acid into a nickel plating bath to minimize nickel oxide formation. His technology was awarded a U.S. patent in 1878.5, 16
Dr. Wilhelm Pfanhauser and Chloride
Working on further improvements to the nickel plating bath in Austria, Dr. Wilhelm Pfanhauser, published in 1900, his work on nickel plating baths wherein ammonium chloride was added to the bath.5
 Dr. Wilhelm Pfanhauser16Dr. Pfanhauser found that the addition of chloride to the bath, aided in nickel anode corrosion. By increasing the corrosion rate he found that more dissolved nickel metal would be available in the bath. This of course increased the efficiency of deposition.5
Dr. Wilhelm Pfanhauser16Dr. Pfanhauser found that the addition of chloride to the bath, aided in nickel anode corrosion. By increasing the corrosion rate he found that more dissolved nickel metal would be available in the bath. This of course increased the efficiency of deposition.5
Dr. O.P. Watts and the Watts Plating Bath
Into this cacophony of emerging research, patent filings, legal patent defense claims, the use of boric acid to maintain pH and the recognition of chloride salts as an anode corrosion enhancement tool, stepped Dr. O.P. Watts at the University of Wisconsin.1, 2, 3, 4, 5, 15, 17, 21
Having received his PhD in 1905 from the University of Wisconsin, Dr. Watts; spent the next 11-years tinkering with; initially cobalt, but later nickel plating baths. His landmark paper; “Rapid Nickel Plating,” was published in the Transactions of the American Electrochemical Society in April, 1916.4, 5 With the publication of this paper the blueprint was laid for the truly modern nickel plating bath, the bath that is most commonly in use around the world today. Today the modern nickel plating bath is known as the Watts Nickel Bath in Dr. Watts’ honor.4, 5, 13, 14, 17
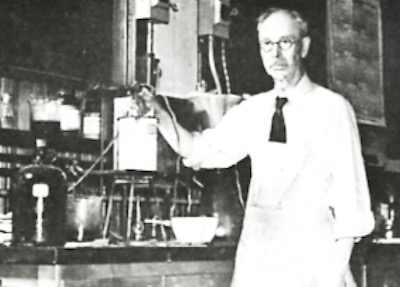 Dr. Oliver P. Watts in his laboratory at the Univ. of Wisconsin.5,16Taking advantage of Weston’s previous discovery of boric acid to maintain nickel bath pH and Pfanhauser’s 1900 addition of chloride salts to foster anode corrosion Dr. Watts’ made two significant breakthroughs. First, he elevating the nickel bath’s operating temperature5 and secondly, he realized if he could substitute nickel chloride for ammonium chloride to maintain good anode corrosion.5
Dr. Oliver P. Watts in his laboratory at the Univ. of Wisconsin.5,16Taking advantage of Weston’s previous discovery of boric acid to maintain nickel bath pH and Pfanhauser’s 1900 addition of chloride salts to foster anode corrosion Dr. Watts’ made two significant breakthroughs. First, he elevating the nickel bath’s operating temperature5 and secondly, he realized if he could substitute nickel chloride for ammonium chloride to maintain good anode corrosion.5
A galvanic cell carries electrical current efficiently when there are sufficient dissolved salts in solution to conduct the current. Using nickel chloride for anode corrosion not only increased the amount of nickel cations available for deposition but additionally maintained the chloride anion for anode dissolution. As a result of these changes, the Watts Nickel plating bath has a much higher level of dissolved nickel salts when compared to the earlier nickel baths of Bottger and Adams.2, 3, 4, 5, 15, 19
Table No. 2
Typical Make-up of a Watts Nickel Plating Bath1, 2, 3, 4, 6, 15, 17, 18, 19, 20
- Constituent: Concentration
- Nickel Sulfate 35 – 40 oz./gal
- Nickel Chloride: 10 – 14 oz./gal
- Nickel Meta: 9 – 10 oz./gal
- Boric Acid: 6 – 7 oz./gal
- pH: 3.5 - 4.0
- Temperature: 140°F
Where:
- The sulfate is the main source of platable nickel
- The chloride is present to aid in anode corrosion thereby replacing cathodically deposited nickel
- The boric is present to buffer the bath’s pH
Elevating the bath’s operating temperature to 140°F; and even as high as 160°F in some applications, coupled with the higher concentration of dissolved nickel salts for electrical efficiency Dr. Watts created a nickel bath that could accept a much higher, applied, DC electrical current without cathode D.C. burn.4, 5, 7, 17 Additionally, the higher concentration of salts permitted an increase of throwing power in low current density areas. By increasing current in the bath Dr. Watts was able to deposit more nickel per hour in his bath; in accordance with Faraday’s First Law.3, 4, 14 Looked at from a different angle, Dr. Watts was able to achieve a desired nickel thickness more rapidly because the bath was more electrolytically efficient.16
Bright Nickel Plating
Semi-bright nickel deposits are bright but not reflective.3, 4 The deposits are white, matte and malleable.3, 4, 5, 13, 19 Prior to the introduction of brightening agents to the nickel plating bath, this matte and malleable nickel deposit would have to be hand-buffed to achieve a mirror-reflective finish. This was prior to the introduction chrome plating to meet the rise in the popularity of automotive bright trim in the 1930s.3, 4, 14, 21
Considering the cost of the hand labor; even in the late 1920s and early 1930s required to achieve the improved mirror-reflective finish, the search was on to develop a nickel bath capable of generating a mirror-reflective appearance as the nickel was being deposited.14
 Max Schloetter16Working at the plating supply company he founded in Berlin, Germany in 1912, scientist and entrepreneur; Max Schloetter, discovered in 1930 that the introduction of the organic aromatic sulfonate compound; sodium benzene disulfonate, would generate a dramatically improved, hard, smooth, mirror-reflective finish to what was formerly a semi-bright nickel plated deposit.2, 3, 4, 5, 14
Max Schloetter16Working at the plating supply company he founded in Berlin, Germany in 1912, scientist and entrepreneur; Max Schloetter, discovered in 1930 that the introduction of the organic aromatic sulfonate compound; sodium benzene disulfonate, would generate a dramatically improved, hard, smooth, mirror-reflective finish to what was formerly a semi-bright nickel plated deposit.2, 3, 4, 5, 14
In 1932, Schloetter filed for a U.S. Patent and in 1933 he was granted U.S. Patent No. 1,972,693 for his bright nickel plating additive.5 He began advertising his bright nickel plating process in assorted German trade journals on December 1, 1933.5 In 1934, Schloetter sold the rights to the Schloetter Process to The Pyrene Manuf. Co. in the U.S. which began marketing the first practical bright nickel plating bath as Pyrene High Gloss Nickel in 1934.5, 14
Also in 1934, Mr. Virgil Waite; with the McGean Chemical Co. filed a patent on the use of aromatic sulfonates coupled with the use of cadmium and/or zinc as brightening agents.6 This development was the first of the bright nickel solutions better known today in which separately added control agents and brightening agents are added to the bath.6
The Electroplaters’ Society
 Assoc. Founder Charles Henry Proctor11, 12On March 6, 1909, Charles Henry Proctor, the plating and foundry supervisor at the F.H. Lovell Co. in Arlington, NJ; achieved his long a dream and inspired a meeting of some two-dozen foremen platers.12, 17
Assoc. Founder Charles Henry Proctor11, 12On March 6, 1909, Charles Henry Proctor, the plating and foundry supervisor at the F.H. Lovell Co. in Arlington, NJ; achieved his long a dream and inspired a meeting of some two-dozen foremen platers.12, 17
Their meeting; held at the old Hotel Chelsea in New York City, was for the purpose of creating a non-profit association to foster the advancement of electroplating, metal finishing and allied arts.12, 17
Out of their thinking and planning the National Electroplaters Association of the United States and Canada was formalized or NEPA, came into being at an organizational meeting held at the same Chelsea Hotel on Saturday, April, 10, 1909.12, 17
For his organization efforts and prescience members selected Charles Henry Proctor; NEPA president. Proctor presided at that first meeting, at which the infant organization approved its constitution and by-laws.17 The 60 charter members at this meeting were a doubling of number present at the meeting; just one month previously. NEPA was incorporated as a New York Corporation on October 18, 1909.12, 17
From its inception, NEPA was a technical-educational society with its principal reasons being:
- “To advance and disseminate knowledge concerning the art of electrode-position of metals.
- To maintain a laboratory equipped for research work.
- To conduct meetings for the purpose of presenting papers on appropriate technical and scientific subjects and
- To publish technical literature.”12
 Chelsea Hotel, NYC, circa 1920. 9, 12The first NEPA Banquet was held January 15, 1910.12, 17 By 1912 NEPA became international chartering a branch in Toronto, Ontario. The NEPAs third annual banquet in 1912 featured the first exhibit of suppliers’ products. It was the precursor to today’s SUR/FIN conferences.16
Chelsea Hotel, NYC, circa 1920. 9, 12The first NEPA Banquet was held January 15, 1910.12, 17 By 1912 NEPA became international chartering a branch in Toronto, Ontario. The NEPAs third annual banquet in 1912 featured the first exhibit of suppliers’ products. It was the precursor to today’s SUR/FIN conferences.16
In 1913; was reorganized. It adopted as its name the American Electroplaters Society or AES. The reorganization was made to further meet its mission and growing membership. At the 1913 Annual Banquet, the AES constitution and by-laws were adopted and George B. Hogaboom was elected first AES president.12, 17
George Hogaboom; a charter member of the society was a Connecticut based foreman plater who is best remembered for co-authoring with Dr. William Blum, the electroplater primer still used today entitled “The Principles of Electroplating and Electroforming.”1
 NEPA logo 1909-1913.16, 17AES remained the legacy society for 72-years and adopted as its logo, the three intertwined AES letters surrounded by the society’s name American Electroplaters Society.
NEPA logo 1909-1913.16, 17AES remained the legacy society for 72-years and adopted as its logo, the three intertwined AES letters surrounded by the society’s name American Electroplaters Society.
By 1985, the society morphed yet again. In recognition of the society’s 75th or “Diamond Jubilee” anniversary and the changing work environment of membership; which had expanded beyond just electroplaters and now consisted of individuals who were involved in all aspects of surface finishing, the society’s name and logo were again changed.16
The society’s new name was the American Electroplaters’ and Surface Finishers Society; or more conveniently just AESF. The new logo included the society’s AESF moniker; in gold font, atop of a stylized blue diamond background; symbolic of the legacy society’s diamond jubilee.16
 AES Logo; 1913–1985.16With the advent of personal computers, the availability of information on line, the diminishing influence of “old timers” and the increase in retirees there came a diminution in societal attendance at the branch and national levels. Falling membership levels and the resultant dues funding lead to a near bankruptcy of the society by the early to mid-2000s. However a dedicated team of AESF loyalists; who understood the historical significance of the society and the camaraderie of fellow membership, were able to reorganize the society into its current format.
AES Logo; 1913–1985.16With the advent of personal computers, the availability of information on line, the diminishing influence of “old timers” and the increase in retirees there came a diminution in societal attendance at the branch and national levels. Falling membership levels and the resultant dues funding lead to a near bankruptcy of the society by the early to mid-2000s. However a dedicated team of AESF loyalists; who understood the historical significance of the society and the camaraderie of fellow membership, were able to reorganize the society into its current format.
In 2007, AESF was reorganized as the National Association for Surface Finishing or NASF, upon a merger with the National Association of Metal Finishers (NAMF) and the Metal Finishing Suppliers Association (MFSA). Both the NAMF and the MFSA, proud legacy equals to the AESF, were suffering from the same membership ills. It seemed and remains a logical merger of the societies all of which have as a common core; metal finishing.16
A new NASF logo was adopted and Ray Lucas from Valley Chrome in Clovis, CA, was selected as the association's first president. In addition, the AESF Foundation was formed to serve as the society’s educational bulwark.

About The Author: William Nebiolo received a B.A. from The University of Connecticut and an M.S. in environmental sciences from Long Island University. He has been with REM Surface Engineering since 1989 and serves as a sales engineer and as product manager. Since 1978, Nebiolo has been an active member in the NASF, where he has represented the Connecticut chapter as an NASF national delegate and is the 2010, 2014, and 2015 recipient of the NASF National Award of Merit. From 1996 to 2000, he served as one of SME’s Mass Finishing technical training program instructors. He has published and presented dozens of technical papers and is the author of the SME Mass Finishing Training Book. Nebiolo can be reached at bnebiolo@remchem.com.
References
1. Blum, William PhD, Hogaboom, G.B.; “Principles of Electroplating and Electroforming”; 3rd Edition; 1949; McGraw-Hill Book Co.
2. DiBari, Dr. George A.; Practical Nickel Plating: An AES Illustrated Lecture, The American Electroplaters’ Society Inc.; Newark, NJ; 1973; pp. 1-30
3. DiBari, Dr. George A., Dr. R.A. Covert; Nickel Electroplating: An AESF Educational Course; The American Electroplaters and Surface Finishers Society; Orlando, FL; 1992; pp. 1-32
4. DiBari, Dr. George A.; Chapter 3 “Electrodeposition of Nickel”; Modern Electroplating; edited by Schlesinger M. Paunaovic M.; John Wiley & Sons, Inc. New Jersey; 2010; pp. 79-114
5. Dubpernell, Dr. George; “The Story of Nickel Plating”; Plating, Vol. (46) 6; 1959; pp. 599-616
6. DuRose, Arthur H., The 11th William Blum Lecture, 57th Annual AES Convention of the American Electroplaters’ Society, June 22, 1970, Montreal, QB
7. Gianelos, Louis; “Troubleshooting of Nickel Plating Solutions (AES Update: Part I of III – Part Series on Nickel); Plating & Surface Finishing; Vol. (64) 8; 1977; pp. 32-34
8. Giorio, B.; www.silvercollection.it Englaprime; Thomas Prime & Sons, 2017
9. Gonne, Maude, New York as it Was, Pinterest web pin, New York City Historic Buildings, 2018
10. Grace’s Guide to British Industrial History, www.gracesguide.co.uk/elkington, website, 2017
11. LaManna, Flavio, J.; The Fundamentals of Chemistry for Electroplaters; Electroplating Course Manual; Chapter 2; The Newark Branch of the American Electroplaters’ Society; 1980
12. Lindsay, James H. PhD, AESF Fellow; Editor Plating and Surface Finishing; 2005, Oct.
13. McKay, R.J.; “The History of Nickel Plating Developments in the U.S.A., Part I,” Plating; (38) 1; 1951; pp. 41-44 & p. 57
14. McKay, R.J.; “The History of Nickel Plating Developments in the U.S.A., Part II,” Plating; (38) 2; 1951; pp. 147-156
15. McMullen, Warren H.; “Chapter 8: Nickel Plating”; Electroplating Course Manual; Basic Practical Electroplating; Edition No. 8; The Newark Branch of The American Electroplaters’ Society; 1980; pp. 110-129
16. Nebiolo, William P.; 2017; NASF New England Regional; Salem, MA; The History of Electroplating
17. Nichols, John P.; Kaleidoscope of AES’s First Half Century; Plating, 1959, Vol. 46, Issue 6, p. 650
18. Oniciu L., L. Muresan; Some Fundamental Aspects of Leveling and Brightening of Metal Electrodeposition”; Journal of Applied Electrochemistry, Vol. (21) 29; 1991, pp. 565-574
19. Read, Harold J., R. Weil; Grain Size and Hardness of Nickel Plate as Related to Brightness; Plating; (37) 12; 1950; pp. 1257-1261
20. Saubestre, Edward B.; “The Chemistry of Watts Nickel Plating Solutions”; Plating, Vol. (45) 9; 1958; pp. 927-936
21. Saubestre, Edward B.; “The Chemistry of Bright Nickel Plating Solutions”; Plating; Vol. (45)12; 1958; pp. 1219-1227
22. The Metal Industry Co., The Metal Industry, Vol. 5, June 1907, p. 167



































