Electropolishing has been in commercial use since the 1950s, and it continues to be a very popular surface finishing process, geared to achieving specific surface treatments on a wide variety of metals.
The net result is the application of electropolishing in many industries. None of which would be able to function optimally without the benefits that electropolishing affords. Let’s break down the process and highlight important aspects.
 Stephen F. RudyBasically, electropolishing replaces traditional mechanical treatments, such as milling, blasting, grinding, and polishing. These often labor intensive operations can be substituted by the economical and expedient electropolishing treatment. This is an excellent procedure to streamline and smoothen microscopic surfaces. Polishing and deburring are accomplished in a single step. Burrs, typically of less than 0.005 inches in size are removed. It allows for greater bulk processing of parts. What you get is a clean, even surface finish. It is one of the best surface polishing methods. Sterilization is enhanced for medical and pharmaceutical applications. Passivation of stainless steels to optimize corrosion resistance is excellent. Other exceptional results include: removal of oxides and tarnish, producing a uniform surface (aesthetic appearance), reducing friction and galling, produces a desired micro hardness, increases magnetism, and improves welding and brazing.
Stephen F. RudyBasically, electropolishing replaces traditional mechanical treatments, such as milling, blasting, grinding, and polishing. These often labor intensive operations can be substituted by the economical and expedient electropolishing treatment. This is an excellent procedure to streamline and smoothen microscopic surfaces. Polishing and deburring are accomplished in a single step. Burrs, typically of less than 0.005 inches in size are removed. It allows for greater bulk processing of parts. What you get is a clean, even surface finish. It is one of the best surface polishing methods. Sterilization is enhanced for medical and pharmaceutical applications. Passivation of stainless steels to optimize corrosion resistance is excellent. Other exceptional results include: removal of oxides and tarnish, producing a uniform surface (aesthetic appearance), reducing friction and galling, produces a desired micro hardness, increases magnetism, and improves welding and brazing.
There are a wide range of industries that make important use of electropolishing. These include: general fabrication, marine, aerospace, automotive, pharmaceutical and medical, petrochemical, food and beverage service, furniture, and appliance. Over the years different electrolytes have been developed that can electropolish many metals and alloys, including: stainless steels (300 and 400 series), aluminum, brass, copper, bronze, nickel, steel, titanium, inconel, hastelloy, gold, silver, and kovar,
How It Works
Electropolishing follows the fundamental principles of Faraday’s Law. It is an anodic treatment of parts that are fully immersed in a special acidic electrolyte. Ancillary equipment, such as bussing, heaters, anode and cathode bars, DC rectifiers, agitation, are all similar to those used in traditional electroplating. With current applied at specific current densities, for a pre-determined time, anodic oxidation dissolves metal off the substrate surface. It is essentially a controlled, accelerated corrosion of the part. Optimum electropolishing is achieved per the traditional operating parameters (current, time, temperature), along with bath chemistry, racking, positioning, and part to cathode distance.
The bath is a mixture of acids, such as sulfuric and phosphoric, along with other agents and stabilizers. A production bath achieves and maintains a concentration range of dissolved metals. A chemical equilibrium forms, with precipitated metals as sludge. The sludge is periodically decanted and some of the electrolyte replaced, to maintain a specific solution specific gravity range. The operating solution specific gravity increases with the accumulation of ampere hours. The electrolyte becomes thicker, taking on the properties of an insulator or resistor. With applied current and focusing on the direct surface to immediate solution interface, the solution film thickness increases, thereby insulating the high current density areas of the part surface. This section of the part becomes less active. In the low current densities, the solution film thickness decreases, resulting in a less insulated, or more active portion of the part. This comparison of surface activities leads to a normalization of microscopic peaks to valleys, dramatically improving the surface leveling characteristics.
Other benefits occur during the electropolishing treatment. Hydrogen is removed from the surface of parts, providing a stress relieving anneal. Oxygen, evolves at the part (anode), forming an agitation medium. This refreshes the electrolyte film surrounding the part. Electrical charges are greatest at edges, or sharpened, irregular points. That is why electropolishing is an excellent deburring application. These areas exhibit greater electromotive potential. In the opposite picture, valleys or low current density areas exhibit less electromotive potential. This electromotive potential controls the electropolishing dynamics.
There is a significant preference to remove high spots on the surface, resulting in a dramatic change of dimensions to high spots. Consequently, there is less change to dimension of low spots. The result is an overall smoothening effect to the metal surface. Compared to mechanical treatments, the dimensional change in electropolishing is small to obtain a desired polishing effect. The overall dimensional change to a part can be 0.00025 inches.
A general rule is that large flat surfaces tend to produce satin or refractive finishes. Smaller parts or those with a radius of curvature tend to produce reflective, mirror finishes. The more curve to surface results in a brighter polishing effect.
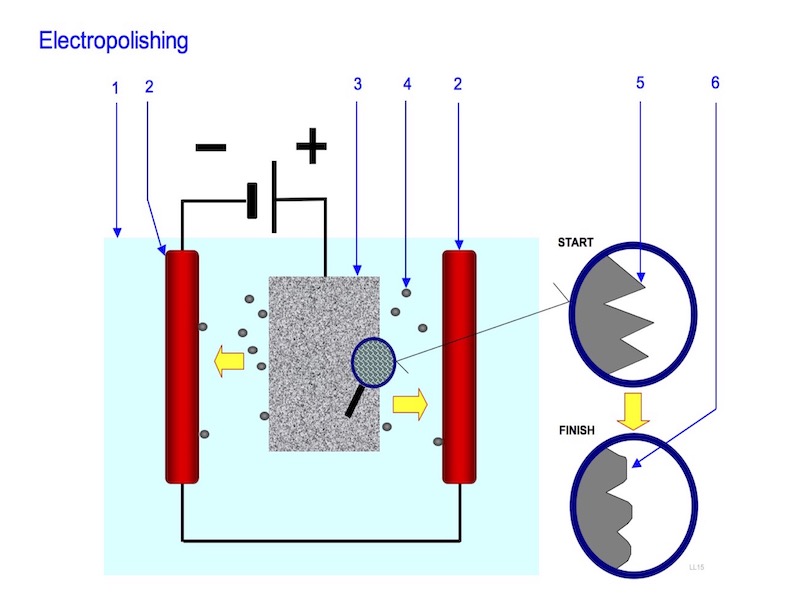
1. Electrolyte; 2. Cathode; 3. Work-piece to polish (Anode); 4. Particle moving from the work-piece to the cathode; 5. Surface before polishing; 6. Surface after polishing
Processing Terms
The following are some terms relevant to electropolishing and post evaluation of the surface of conditioned parts.
- Surface Roughness. Ra (roughness average) or RMS (root mean square) is measured in micro inches. It is a quantitative measure of smoothness of finished surfaces.
- Surface Chemical Analysis. A determination of contaminant levels or the effect of decontamination, resulting in desired surface purity.
- Surface Chromium Enrichment. For passivation, where the chromium concentration exceeds iron, as a ratio in the surface layer. The focus is corrosion improvement.
General Operating Parameters
|
Temperature |
80 – 220 degF (27-104 degC) |
|
* Equipment |
Acid resistant materials |
|
Exhaust |
Required |
|
Power Supply. |
DC Rectifier, 6-20 volts |
|
Agitation |
Solution movement (circulation pump or air) |
|
Heating |
Steam coils, electric immersion |
|
Cathodes |
Copper, lead, or stainless steel |
|
Racks |
Copper, titanium, or plastisol coated copper with titanium tips |
* Tanks for stainless steel electropolishing may need to support the electrolyte solution of approximately 14 lb/gal. For example 800 gallons of electrolyte would weigh approximately 11,200 lb.
The power supply should be protected from any close proximity to the corrosive EP solution, as should all ancillary equipment.
For effective racking, a guide to use is 0.001 square inch of copper carries approximately one amp of current.
The above information is an example of a typical set up for electropolishing stainless steel. Racks and bath temperature may be modified to fit the requirements of processing other metals and alloys.
It is important to acknowledge all the safety information that is provided by suppliers with respect to handling, storage, and transporting electropolishing solutions. This includes protective clothing and understanding related documents, such as technical bulletins and safety data sheets (with copies in the office and in the OSHA Right to Know binder). Line process tanks should be properly labeled as per the local, state, or federal requirements.
Typical Operating Parameters for Stainless Steel Electropolishing
|
Temperature |
130-180 degF (54-82 degF) nickel alloys |
|
Temperature |
190-210 degF (88-99 degC) non-nickel alloys |
|
Temperature |
150-180 degF (66-82 degC) non-nickel alloys |
|
Current Densities |
150-450 amps/ft2 |
|
Operating Voltages |
6-18 |
|
Cathode to Anode (parts) ratio. |
10:1 to 1:1 |
|
Electropolishing time |
3- minutes (as typical) up to 20 minutes |
|
Maximum current input |
5 amps per gallon |
|
Cathode to parts distance |
2-6 inches |
|
Parts from tank bottom |
At least 6 inches |
During EP (electropolishing) treatment, the metal removal rate may range from 0.00005 to 0.0001 inches per exposed surface per minute of electropolishing time. This is dependent upon operating current densities.
Distance from cathode to part is critical to provide the preferred chemical polishing action. A smaller separation distance may result in etching and pitting.
It is important that racked parts on the bottom row be at least 6 inches above the tank bottom. EP develops metal sludges during operation. The recommendation minimizes contact of parts in sludge (as long as proper bath maintenance is conducted). Surpassing the maximum current input will overheat the electrolyte, at which point a cooling source, such as a coil would be required. The actual voltage required is based on the bath temperature, workload, shape of parts, surface treatment requirement, and distance of parts from the cathodes. EP exhibits poor throwing power that is why relatively high current densities are required.
Typical Process Cycle
- Degrease or Soak Clean
- Double Rinse
- Optional Descaling
- Double Rinse
- Electropolish
- Drag Out
- Double or Triple Counterflow Rinse
- Dry
A surface clean of oils and grease is very important. Electropolishing will not remove these types of organic soils. Descaling is optional, since EP provides good descaling capability. Post EP rinsing is important, not only to wash the thick electrolyte off parts, but to minimize metal dissolved waters in waste treatment. To facilitate the rinsing of complex shapes or where traces of dilute acid electrolyte remain, a mild alkaline neutralization dip in a soda ash solution is helpful. Afterwards, rinse in clean water. After processing, stainless steel parts normally do not require any additional treatments.
It is important to understand that the electropolishing solution is hydroscopic. Specifically, it readily absorbs moisture from the surrounding air. As the concentration of water increases, the desired specific gravity of the bath electrolyte is affected. Therefore, it is highly recommended to cover the tank when not in use.
Analysis and Maintenance Control
Most analysis control methods require a few standard procedures to maintain optimum chemical balance of the electrolyte. These can be summed up as follows.
- Titration of Acids. As an example, stainless steel EP electrolytes contain a balance of at least two inorganic acids, in addition to other supporting additives. The acids can be analyzed by titration, using a two-step procedure, involving different pH endpoints. Upon analysis of each acid, a ratio is determined. Maintenance or corrective additions can be made as the electrolyte concentrate or separate acids (as required).
- Specific Gravity. Where the electrolyte is in a recommended range for the operating bath, at a given solution temperature. The specific gravity measurement includes the total of dissolved metals and water. This direct reading is an approximation of these bath constituents. It should be measured daily if the bath is used in continuous production. The data enables one to appropriately adjust the bath (or replace a portion of it), by addition of concentrated electrolyte and water, as required. As an example, depending on the specific alloy electropolished, the decantation may be 5-10 gallons per 10,000 to 15,000 amp hours.
- Analysis of Dissolved Metals. This is tied into specific gravity. As the EP bath accumulates ampere hours of operation, the concentration of dissolved metals will increase. As this progressively occurs, the electrolyte specific gravity will increase, as will the tendency for sludging. Some EP treatments are adversely affected by the buildup of some metals, such as iron in stainless treatments. Analysis by Atomic Absorption is recommended to determine the quantitative concentrations of key members such as Iron, Copper, Nickel, and Chromium. There will a point at which electrolyte replacement is recommended, sometimes on an ampere hour basis. This replenishment is important to maintain desired EP surface treatment results.
- Hull Cell Method. Using a standard porcelain, 267 milliliter hull cell, at bath operating temperature and a lead or stainless steel cathode, the stock stainless steel anode panel is electropolished. Time and current setting commensurate to processing parameters in the tank. For example, 2-3 amps for 3 minutes. Afterwards the electropolished panel is thoroughly rinsed and examined using a hull cell ruler guide to determine effects at specific electropolishing current densities.
Analysis control is not complicated, but it is important should not be overlooked or ignored. As with any metal finishing process, quality finishes are heavily based on quality control of the process baths. Combine this with operating per the optimum parameters, and satisfactory EP treatments will be routine and continuous.
Waste Treatment
As previously described, the EP solutions are typically mixtures of mineral acids. These acids are readily neutralized by adding alkali (typically 50% Caustic Soda) in a waste treatment system. However, there will be dissolved metals, such as Chromium, Copper, Iron, and Nickel, that require specific treatment to precipitate, coagulate and flocculate. It is important to establish whether or not metal complexing agents are part of the EP solution. This may require additional consideration in the treatment process. Jar testing is typically used to determine additions of specific additives to achieve the required effluent quality for compliant discharge. The predetermined process is then incorporated into the operating waste treatment system.
Problems in the Process
|
Problem |
Correction |
|
Insufficient treatment. |
Adjust operating parameters, bath chemistry as required |
|
Pitting |
Check cleaning, racking, agitation, bath volume |
|
Staining |
Rinsing poor, inadequate, slow, contaminated |
|
Gas patterns, streaks |
Adjust voltage, realign racking of parts, solution agitation |
|
Dull or leopard spots |
Improve cleaning before EP |
Other problems related to the process include, mechanical and electrical (loose bussing or rectification breakdown). The intended application could be one selecting the wrong electrolyte or the EP treatment may not be right.
Limitations
Electropolishing may not conceal or cover surface defects, such as non-metallic inclusions or seams. Rough or aggressive scratches may not be overcome. Certain defects, such as: pits, grains, or dullness that are extreme, may not be overcome. Heavy orange peel or mold surface texture do not lend to EP. Alloys should be confirmed for compatibility. For example, multi phase alloys promote a difference in the anodic EP treatment. Cast metals, due to the extent of porosity, do not lend well to EP.
Electropolishing Overall
This unique metal finishing operation provides an economical, resilient, easy to use process. It rapidly develops a level and bright surface finish on many metals. Such metals include Stainless Steels (most popular), carbon steel, steel, nickel, nickel silver, aluminum, and brass. Formulated and generic electrolytes effectively condition metals to meet the treatment requirements. The electropolished metal is effectively: deburred, descaled, polished, passivated, size modified, establishing the desired surface finish. An extremely clean and contaminant free surface is achieved.
Since it’s initial development in 1929, the application of electropolishing has expanded to many industries, serving the commercial, medical, military, aerospace, automotive, and consumer markets, to name the major ones. Through the ensuing decades improvements and refinements have lead to electropolishing being a major contributor to effective metal finishing.
Stephen F. Rudy, CEF, is president of Chem Analytic, and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.