All plating baths are similar in that a basis metal or previous metal electrodeposition is to be covered with a new layer of a specific metal.
 Stephen F. Rudy CEFEvery such bath is water-based, operated within some specific temperature range, be it on a cold, warm, or hot profile. Filtration is almost universally accepted as very important to maintenance, purity, and process operation. Anodes are mostly of the metal to be plated, with some applications for inert types. Agitation is normally required, be it mechanical or by air.
Stephen F. Rudy CEFEvery such bath is water-based, operated within some specific temperature range, be it on a cold, warm, or hot profile. Filtration is almost universally accepted as very important to maintenance, purity, and process operation. Anodes are mostly of the metal to be plated, with some applications for inert types. Agitation is normally required, be it mechanical or by air.
When things go wrong, it has many times been something overlooked or not properly set for the specific process. What to do? What resources are available to us? Where do we look or check? To get a handle on questions and add some other tips, let us consider items that can quickly focus on, resolve, and minimize re-occurrences of problems.
Support Sources
Suppliers, vendors, finishers, and related educational sources provide technical expertise for their marketed plating processes. They can be a wealth of practical information and technical service. Technical bulletins provide operating parameters, procedures, maintenance, analysis. Many include comprehensive troubleshooting guides. Often it occurs that a basic analysis will confirm one or more bath concentrations to be off (deficient or in excess), operating parameters such as pH or temperature to be out of range. Appropriate corrections usually result in plating improvements. Breaking down what to check or look for in times of plating trouble, here is a good starting point as past corrective actions have and continue to be helpful:
- Basic wet analysis
- Hull cell evaluation (is the acknowledged problem reproduced)
- Operating parameters
- In Plating Baths
- Current application and voltage
- Basis metal preparation and previous steps to the problem bath
- Rinsing
The Hull cell was invented and patented in the 1930s by Dr. R. O. Hull. This troubleshooting and analysis tool has been highly valued as a dependable resource for plating solutions.
267-milliliter hull cell
With regards to troubleshooting, the hull cell is paramount to identifying a problem and the source for corrective action. In almost all plating tests, the hull cell is shown to accurately highlight the problem. If, for example, a nickel bath has been contaminated with zinc, this manifests as a dark grey, streaked low current density deposit. In this case, dummy electrolyzing will adequately purify the solution.
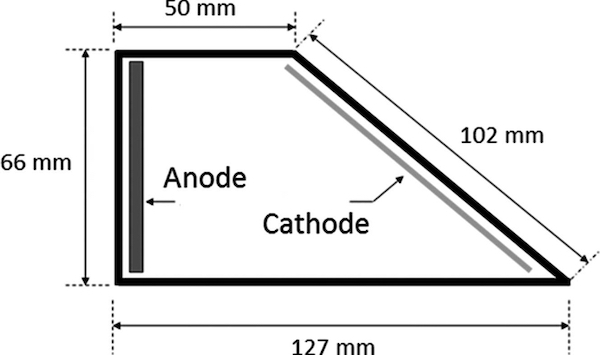
The hull cell can be used as an effective tool to also determine how much dummy electrolyzing is required to, for example, purify a 500-gallon rack nickel tank back to production status. First, plate the hull cell panel at full amperage (2 amps for 5 minutes) with appropriate agitation. Then, upend the panel, placing it in the 127 mm area in the previous hull diagram. Position the nickel anode directly opposite in the 50 mm position of the hull cell diagram. Reduce the current to 0.15 amps, representing 5 amps/ft2. This setup is now used to dummy electrolyze the panel. Observe the panel periodically until the low current density confirms a preferential deposition of white nickel. In the example, it requires 2 hours of dummying. Now, to scale up from the 267 milliliters (0.0705 gallons) solution to the 500-gallon tank:
- A 2” X 2” (0.028 ft2) section of the panel (cathode) was plated at 0.15 amps X 2 hours to yield 0.3 amp-hours per 0.0705 gallons.
- 5 amp-hours / 0.0705 gallons X 500 gallons = 2,128 amp hours of dummying in the 500-gallon nickel tank to restore full white nickel deposit to the exposed dummy cathode.
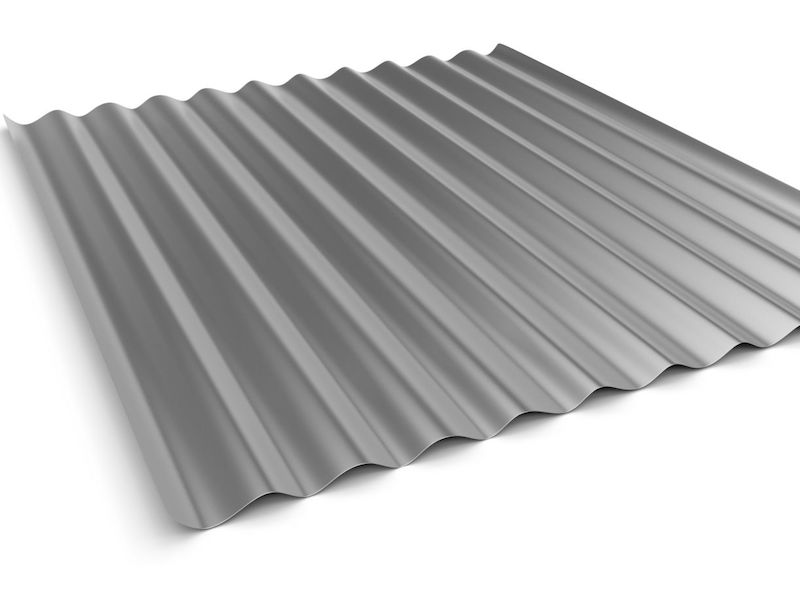
Corrugated steel sheet
The dummy should be a clean steel corrugated sheet. This dummy configuration is used as zinc preferentially deposits in the low current density area. Measure the surface area of one corrugation. Multiply this surface area by each of the rest of the corrugations. Having this product, multiply by 2 (the dummy sheet has two sides) to get the total surface area of the dummy sheet. Next, copy the hull cell procedure by first plating the entire dummy sheet with nickel at 40 amps/ft until it is 100% covered with nickel deposit.
Then, set the dummy amperage to achieve 5 amps/ft2. Check the dummy sheet periodically for preferential white nickel deposition in the inner corrugations. This should, by our example, occur around 2,100-2,200 amp-hours. Selection of dummy sheet size and surface area is important to how long it calculates to adequately purify the zinc contaminated nickel plating bath. If the dummy total surface area were 20 ft2, at 5 amps/ft2, the current requirement would be 100 amps.
- 2,200 amp hours = 100 amps X (hours to dummy electrolyze)
This example calculates to 22 hours of dummy plating at the settings as described.
It is important to check any and as many of the processes associated with the specific plating problem. Frequently, the problem(s) with corrections can be readily identified early on. Be sure to evaluate as many of the contributing processes, since some problems are the result of more than just one or two other factors. As an example, plated parts have a haze and brittle deposit instead of the expected bright and leveled appearance. The problems were found to be insufficient cleaning and excess brightener in the plating bath. Simply put, poor cleaning not seen was supposedly compensated for by adding more brightener in the plating bath. This resulted in a brightener excess that caused deposit brittleness. The example problem just given also provides a very important fact that should be considered by every finisher and supplier:
The Best Definition of Poison is Too Much
If an addition of a maintenance additive or corrector seems to help, do not overdose. Sometimes the proposed addition may only serve as a temporary masking effect, unfortunately exacerbating the original problem. This, in turn, can turn the first problem into a veritable monster. While we are considering basics, do not fall into the trap that many unfortunately do so:
You Cannot Violate Faraday’s Law
This is why it is a Law. Do not try to push or deposit more metal than the process is capable of doing so. One of the first lessons I learned in the industry is that it is OK to destroy a plating bath in the Hull cell by evaluating additions and treatments if this is the best initial approach. Then, if proprietary product additions are required, consider first adding half of what is calculated as a requirement. It is immensely easier to add to the bath instead of removing an overdose. After completing the required purification treatment, corrective additions, plating usually resumes on a trial basis while deposit characteristics and post-analysis are verified.
Electrical and Mechanical
Many times these supporting operating units can be overlooked or their importance not confirmed. Equipment is susceptible to malfunction and breakdown. Here are some useful tips and checks that can routinely keep the plating process optimized:
Check rectifier for output and excess AC ripple.
- Make sure bus bar connections are sufficient and not corroded.
- Clean all electrical contacts
- In barrel lines, the insulated electrical lines should be warm, not hot.
- Trace bus connections from the rectifier, confirming correct connection to anode and cathode. Reversing polarity connections can and will occur.
- In many return type or cycle master plating machines, the plating entry should be live. This is to prevent a bipolar effect.
- The previous example also highlights a problem where one contact on the return type plating machine is lost. The live contacts on either side of the dead one cause the dead one to take on a positive charge by induction. This results in deposit etching and passivation.
Check operation and maintenance requirements for the plating bath filter. The manufacturer’s recommendation for operation, packing of carbon and filter aid, and replacement, should be adhered to. The plating tank should be checked for dropped parts. Remove them as soon as it is possible. Corroding parts contribute to metallic contamination of the plating bath and can also result in another source of the bipolar condition.
Anodes are another potential problem source. It is common for plating problems to be associated with anode baskets to be half full or less so. Acknowledging the higher prices for metal anodes is attentive to any seeming good price deals. A certificate of analysis should be provided with any quote or sale. The purity of anodes is very important. Trace metallic impurities can be a real problem. Another item to strongly consider is the physical appearance of anodes. For example, ball anodes of zinc and cadmium have a similar appearance. Don’t get them mixed up. Remember that oxygen-free copper anodes are for application in cyanide copper plating baths. On the other hand, copper phosphorous anodes are applied in acid copper plating baths.
Probably the best troubleshooting sequence is setting up and maintaining a good maintenance process. This, in turn, tends to minimize problems that may occur when maintenance is non-existent or lax. What to do is easy to identify and implement. Reviewing the tips in this article, acknowledging practical recommendations, and taking into account corrective actions to past problems, should provide good maintenance procedures and scheduling. Most important is minimizing plating problems. Rejects can be very expensive and significantly affect the desired production output. We are approaching the end of year point where typical maintenance and purification procedures come into focus. Eliminating plating problems should be the driving force to maintain success.
Stephen F. Rudy, CEF, is president of Chem Analytic, and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.


































