The corrosion and wear resistance of organic coatings are the key factors to maintain their good function.
Graphene-based polymer composites have attracted incredible attention owing to their superior physicochemical properties than polymers. In this study, the effect of graphene nanosheet (G) additives on the microstructure, adhesion behaviors, anti-wear performance and corrosion resistant of epoxy powder coatings (EP) were systematically investigated. Field emission scanning electron microscope (FESEM) results verified that the mild addition of G could enhance the compactness of EP and reduced microstructure defects such as cracks, cavities, and voids. The adhesion and tribological results confirmed that the graphene-contained epoxy powder composite coatings (G/EP) could demonstrate significant improvement compared to the EP, which potentially attributed to the high mechanical and self-lubrication properties of G nanoflakes. In particular, the electrochemical and salt spray evaluation revealed that the enhanced physical barrier protection performance and outstanding anti-corrosion capacity of the G/EP composite coating containing 0.4 wt% G. The current study provides a promising metal protection strategy for the development of graphene-enhanced organic powder coatings applied in mechanical-corrosion coupling environments. This article reveals for the first time that graphene nanosheets enhance the interfacial adhesion strength of organic powder coatings, as well as the effect on wear and electrochemical performance.
1. Introduction
In recent decades, epoxy powder organic coating has been applied extensively as surface functional protective coating for metal substrates due to its good decoration, excellent adhesion, resistant to chemicals, anticorrosion and anti-wear performance [[1], [2], [3], [4], [5], [6], [7]]. More importantly, compared with liquid organic coating, powder organic coating has no volatile organic compounds (VOC) release and less waste during preparation [6,8,9]. It can actively respond to carbon neutrality policy, and its usage has been increasing year by year. The most critical challenge in epoxy powder coatings market is to provide high-quality products at the lowest possible price [2,3,6]. Meanwhile, it is urgent to achieve higher functional of epoxy powder coating to enhance its service lifetime in aggressive environments. In fact, some researchers have used rubber elastomers, thermoplastic resins, nanoparticles introduced into epoxy matrix to improve its mechanical properties, anti-corrosion behavior and wear resistance [2,8,10,11]. Sharifi et al. [12] reported that the epoxy powder coating containing nano-clay (Closite 30B) exhibited better mechanical and anticorrosion performance in comparison to that for neat epoxy powder coating. Fernandez-Alvarez et al. [13] found that nano-addition of SiO2 particles could improve the scratch resistance and anti-wear of epoxy organic powder coating on steel substrate. The epoxy resin improved with 0.75 wt% hydrophilic SiO2 nanoparticles own the best wear resistance, which was mainly related to the enhanced cross-linking of matrix in the coating caused by SiO2 [8]. The hardness and impact resistance of epoxy powder coating could be improved by adding calcium carbonate nanoparticles [2,14,15]. In addition, the addition of alumina, TiO2 and fumed silica micro- and nano-sized particles also enhanced the adhesion to substrate and mechanical properties of powder coatings [16].
Graphene is a single carbon layer of an idealized form of graphite. In the past decade, graphene materials have become extraordinary high-performance additives for organic coating systems due to their high mechanical strength, huge specific surface area, unique microstructure and tiny particle size [[17], [18], [19], [20]]. There is a large amount of literature on physicochemical properties of liquid coatings reinforced by graphene and graphene-related materials [21]. Liu et al. [22] reported that graphene epoxy composite coatings present outstanding barrier properties on H2O molecules compared to epoxy coatings. Open circuit potential (OCP), Tafel and electrochemical impedance spectroscopy (EIS) analysis confirmed that the corrosion rate of composite coating with 0.5 wt% G was one order of magnitude lower than that of epoxy coating. Zhang et al. [23] revealed that the Young's modulus and thermal stability of epoxy coatings of containing 0.7 wt% graphene-based additives were significantly improved (increase by 213% and 73 °C), respectively. Electrochemical and immersion corrosion experiments in NaCl solution showed that graphene epoxy composite coatings own better corrosion resistance than the epoxy resin coatings. Campo et al. [24] demonstrated that the addition of G greatly improved the hardness and anti-wear properties of epoxy resin, which mainly due to graphene acts as a lubricant from the inside of the epoxy resin. Zhang et al. [25] found that the G can greatly enhance the microhardness, thermal conductivity and thermogravimetric properties of epoxy coatings. Under the room temperature condition, the friction coefficient and wear rate of the epoxy coating with 4.0 wt% G were about 80% and 76% lower than that of the epoxy coating, respectively. Li et al. [26] prepared thermosetting powder/graphene oxide (TP@GO) composites by ball milling and reported that the 1.5 wt% GO coating (TP@GO15) own superior corrosion resistance after 9 days of immersion in 3.5wt % NaCl solution, and compared with the pure thermosetting powder coating, the corrosion current decreased by 74.1% and the corrosion potential increased by 34.2%.
However, according to the best of knowledge, there are few publications regarding the use of G to modify solid epoxy resins for powder coatings. In present work, epoxy powder coatings with various concentration of G nanosheets were fabricated on Q235 steel substrates via a mechanical mixing method. The microstructure, adhesion properties, tribological performance and corrosion resistant of graphene epoxy composites powder coatings (G/EP) were systematically investigated. The main aim of the present work is to provide insights for the preparation of high-quality organic powder coatings with excellent adhesion and wear-corrosion performance on carbon steel in harsh mechanical-corrosion synergistic damage environments.
2. Experimental section
2.1. Materials
Alcohol and acetone were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemical reagents were used as received without purification. Epoxy organic powder coatings were supplied via Zhongshan Hizinco Materials Science & Technology Co., Ltd, China. Q235 steel plates (150 mm × 70 mm × 0.8 mm) were provided by Jiangsu Guoqiang Galvanizing Industrial Co., Ltd, China. Graphene was purchased from Zhongshan Hizinco Materials Science & Technology Co., Ltd, China. Tinplate (150 mm × 70 mm × 0.28 mm) substrates were purchased from Biuged Laboratory Instrument (Guangzhou) Co., Ltd, China.
2.2. Fabrication of composite coating
Degreasing Q235 steel plate and the tinplate substrates were ultrasonically cleaned successively in ethanol and acetone for 20 min to remove contaminants on the surface. Tinplate samples were used to evaluate the impact resistance of the powder coating. The G micro-flake powder was uniformly dispersed into the received epoxy resin powder under mechanical stirring. After that, the evenly mixed graphene-contained epoxy composite powder was sprayed on the surface of Q235 steel plate and tinplate substrates using an electrostatic spraying equipment (WAGNER PEM-X1, Germany). Then the coating samples was placed in oven and cured at 210 °C for 10 min, control the thickness of the all as-prepared coatings to be consistent in the spraying process. The prepared composite coatings were epoxy coatings containing 0.2, 0.4, 0.6 and 0.8 wt% G, which were abbreviated as G0.2%/EP, G0.4%/EP, G0.6%/EP and G0.8%/EP, respectively.
2.3. Coating characterization
Field emission scanning electron microscopy (FESEM, SU8220, Japan) and scanning probe microscope (SPM, Multimode 8, Bruker) were used to observe the morphology of G. FTIR (Nicolet IS50, Thermo Fisher Scientific, USA) and Raman spectroscopy (HJY LabRAM Aramis, Horiba Jobin Yvon, France) were applied to characterize the chemical structure of the G, EP and G/EP samples. The surface and cross-section morphologies of the as-prepared powder coatings were determined via SEM (FEI NOVA NANO, USA). The static water contact angle of the all samples were measured via OCA20 contact angle analyzer (OCA20, Dataphysics, Germany). The adhesion behavior of the coatings was assessed by pull-off and Machu test. The impact resistance of the as-prepared samples was evaluated by Gardner impact test (direct and reverse falling dart).
Reciprocating ball-on-disk tribometer (CETR, UMT-3MT, USA) was used to evaluated the tribological properties of the samples. A commercial steel balls with a diameter of 6 mm were applied as the counterpart due to its high hardness. The friction experiments were performed at room temperature with the constant normal load of 5 N (maximum Hertzian contact pressure of about 1.14 GPa), the sliding stroke of 5 mm and the contact frequent of 5 Hz. The rub test period was set to 60 min, during which the coefficient of friction (COF) was continuously recorded. The total sliding distance was 180 m. After that, the morphologies and depth profiles of wear tracks were observed via optical microscope (OM, Leica, Germany), SEM, three-dimensional optical profilometer (RTEC UP Dual Model, USA) and Alpha-Step IQ profilometer to evaluated the wear mechanism. The Kdouble bondV/FS equation was used to calculate the wear rate of the neat EP and G/EP composite powder coating, where V was the wear volume, F was the normal load and S was the sliding distance.
The electrochemical performance of as-fabricated powder coatings on Q235 steel substrates were carried out by an electrochemical workstation (CEI660E, China) with a typical three-electrode system. The corrosive medium was 3.5% NaCl solution. The samples with an exposed area of 1 cm2 was using as the working electrode, saturated calomel electrode was utilized as the reference electrode and platinum plate was employed as the counter electrode. The electrochemical impedance spectrum (EIS) data at different time intervals were detected at a frequency range of 10 mHz–100 kHz. Furthermore, the EIS information were fitted and analyzed via ZSimpWin software with corresponding equivalent circuit models. The potentiodynamic polarization measurements was performed from −1.4 to 0.4 V at a scan rate of 0.01 V/s. Salt spray test was employed to evaluate the corrosion resistance of the as-prepared powder coatings, which was conducted in a neutral sodium chloride salt spray experiment box according to the ASTM B117 standard. All experiments were carried out three times in this paper to check the repeatability.
3. Result and discussion
3.1. Morphology and structure
Fig. 1(a and b) showed the SEM morphology and SPM topography of G. It can be clearly seen that G sheet was almost transparent, indicating it owned a very thin lamellar structure [27]. The thickness of G was measured with SPM (as illustrated in Fig. 1b), and result revealed that the thickness of G flake was in the range of 1–2 nm, indicating the G flake possesses 3–5 layers structure. In addition, it can be observed from Fig. 1(c and d) that the cross-sectional morphology of the EP powder coating displayed many disordered microcracks and pinhole defects, numerous holes and cavities present on the surface, indicating a loose structure. After the 0.2 wt% G was introduced, the cross-sectional morphology become compact, and defects on the surface were reduced (Fig. 1(e and f)). The addition of 0.4–0.6 wt% G nanosheet significantly enhanced compactness and smoothness of the cross-sectional microstructure of the composite powder coating, and there were almost no defects such as voids, cavities and micropores on the surface of coating (Fig. 1(g–j)). However, when the G concentration rose to 0.8 wt%, more pinhole and irregular cracks inside the composite coating, and the surface cavities and pores apparently increase (Fig. 1(k, l)). This was potentially due to the agglomeration caused by excessive concentration of G, leading to defects in the coating [27,28]. Furthermore, the static water contact angle (WCA) results exhibited that the WCA values of the all samples were approximately 76°–80°, suggesting that the incorporation of G nanosheets did not cause variation in the surface free energy of the epoxy powder coatings.
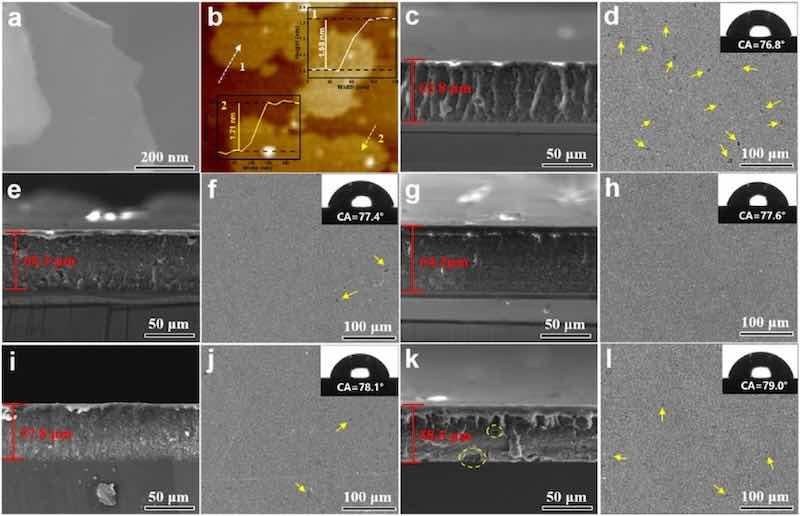
Fig. 1. (a–b) SEM and SPM images of G; the SEM cross-sectional and surface morphologies, water contact angle of the (c–d) EP, (e–f) G0.2%/EP, (g–h) G0.4%/EP, (i–j) G0.6%/EP and (k–l) G0.8%/EP powder coating.
Fig. 2a showed the FTIR spectra of G, EP, and G/EP with various G content. With respect to G, the characteristic peak was centered at 3460 cm−1 corresponding to the stretching vibration of C–OH bond, and the characteristic peak at 1640 cm−1 was attributed to the stretching vibration of Cdouble bondC bond [29]. The typical FTIR characteristic spectral lines of EP was also displayed. Moreover, it can be found that as the concentration of G increases, the characteristic peak of G/EP at 1640 cm−1 (Cdouble bondC bond) was gradually obvious, indicating a well mechanical mixed of the G and EP. Raman spectra result demonstrated that G own three typical characteristic peaks, which were located at 1340 cm−1 (D peak), 1580 cm−1 (G peak) and 2680 cm−1 (2D peak), respectively [30]. The D peak reflects the surface defects and disorder of graphene, the G peak represents the conjugation degree of carbon atoms on the graphene surface, and the 2D peak corresponds the degree of stacking [27,31]. Pure EP does not possess these characteristic peaks. It can be clearly seen that three characteristic peaks of G appeared in the G/EP samples, and the intensity of characteristic peaks of the G/EP composite samples gradually increased with the increase of G concentration. Combined with the results of FTIR, it was confirmed that G/EP composite samples was successfully prepared.
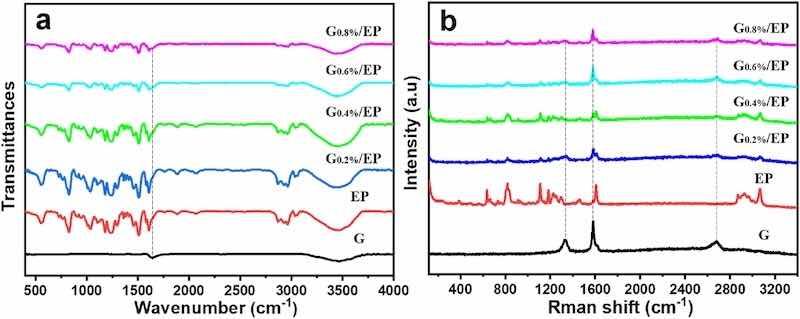
Fig. 2. FTIR (a) and Raman spectra (b) of the G, EP, G0.2%/EP, G0.4%/EP, G0.6%/EP, G0.8%/EP samples.
3.2. Adhesion properties
The adhesion strength is a critical factor affecting the wear and corrosion resistance of the organic coating. The results of the EP and G/EP composite coatings after pull-off, Machu and Gardner impact tests were shown in Fig. 3. After the pull-off test, the EP powder coating present a large area of peeling off, suggesting the inferior adhesion behavior. In the case of G/EP composite coating, no delamination and failure occurred after drawing with epoxy adhesive agent, indicating that introduce of G nanosheet can improve the bonding strength of the powder coating. However, high G content will reduce the adhesion of the coating. With respect to Machu test, the EP powder coating was distinctly observed to almost peel off completely after three times tape tears, exposing a fresh steel substrate and displaying poor bonding strength between the coating and the steel. For the composite powder coating, the surface of the G0.2%/EP, G0.4%/EP, and G0.6%/EP samples were relatively flat, and exfoliation phenomenon does not appears, implying a superior adhesion performance. However, much delamination was observed on the surface of the G0.8%/EP composite coating, indicating that the addition of high concentration G result in a downward trend in the bonding strength of the powder coating. In the case of Gardner impact test, it can be seen the EP powder coating exhibited delamination under direct and reverse falling dart, and circular cracks appeared around the impact region. It is interesting that the addition of mild G significantly enhanced the impact resistance of the powder coating, and the coating remained intact. The poor adhesion and impact resistance of the EP sample are potentially related to its loose structure.
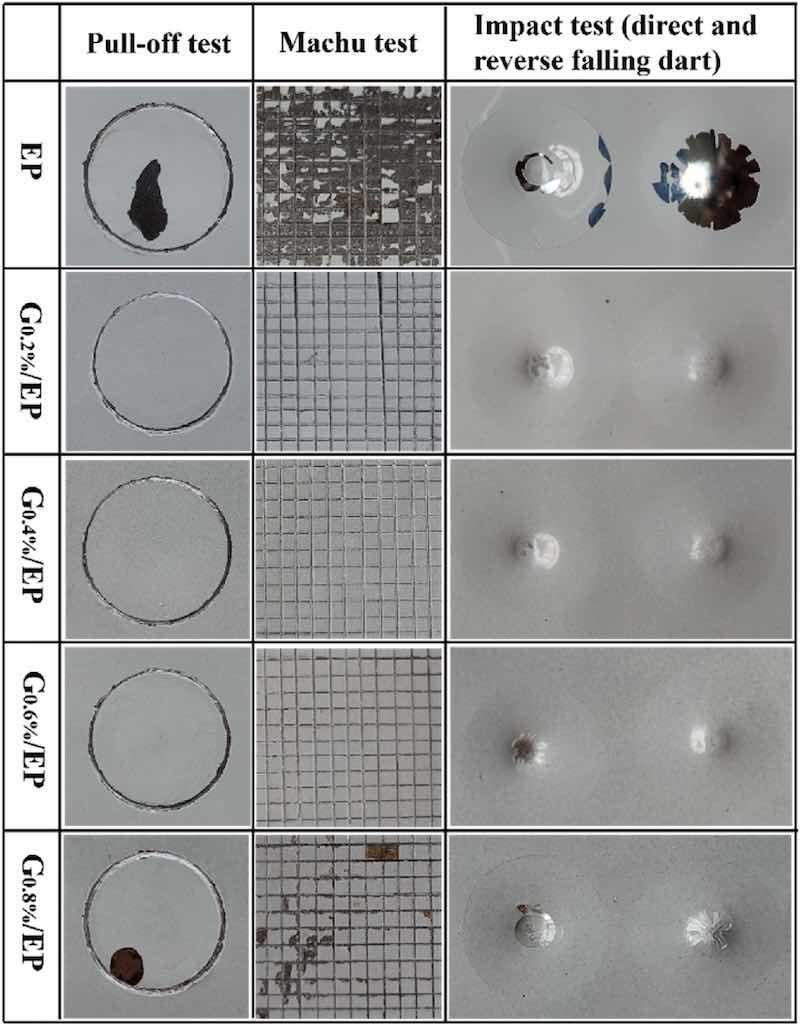
Fig. 3. Digital images of the pull-off, Machu and Gardner tests of the as-fabricated coatings.
3.3. Tribological behavior
As for mechanical moving parts, friction and wear are inevitable during operation [[32], [33], [34], [35], [36]]. The coefficient of friction (COF) and wear rate of the as-prepared coating in 3.5% NaCl solution were shown in Fig. 4a. It can be found that the powder coatings all present similar friction running-in curves. This is due to the contact surface is relatively smooth at the initial stage of friction test, which resulting in a low COF value of the samples. As the rub experiment continues, the deformation of the powder coating with weaker elasticity leads to an increase in the interface roughness. As a consequence, the local effective contact area is decreased, and it is easy to induce stress concentration and uneven deformation in a small region, which increases the COF value [27,28]. Similar trends were also observed in other literature [5,8,9]. In the case of the EP sample, the friction coefficient increased sharply before 1500 s, then slowly rises after 1500 s, and finally fluctuated at about 0.2. A high fluctuation value indicates that the coating may partially failure. With respect to the G/EP composite powder coating, friction coefficient curves were gradually increase before 1200 s, and then remained stable. The G/EP composite coating demonstrated a lower COF values than the EP coating, revealed that the incorporation of G nanoflakes improves the self-lubricating performance of the epoxy powder coating. This was potentially attributed to the lubricating effect of G nanosheet and its good dispersion in the composite coatings [28]. Other similar research results also reported that the addition of 10 wt% SiAlON and 1 wt% of hydrophilic silica nanoparticles could reduce the COF values of epoxy powder coatings from about 0.4 to 0.25 and 0.5 to 0.4, separately. Fig. 4b showed the wear rate of the EP and G/EP composite powder coatings. The EP sample own the highest wear rate of 25.11 × 10−5 mm3/N·m. When G nanoflakes was added in the coating, the wear rate of the G0.2%/EP composite coating was significantly reduced to 6.19 × 10−5 mm3/N·m, and the G0.4%/EP composite powder coating displayed the lowest wear rate of 4.95 × 10−5 mm3/N·m, which was 80.29% lower than that of the EP sample. This is potentially due to the enhanced adhesion strength of G/EP composite coating, which improves its wear resistance. With the increase of G content, the stable wear rates of the G0.6%/EP and G0.8%/EP samples were 6.02 × 10−5 mm3/N·m and 7.11 × 10−5 mm3/N·m, respectively, indicating that excessive G will increase the wear rate of the composite powder coating.
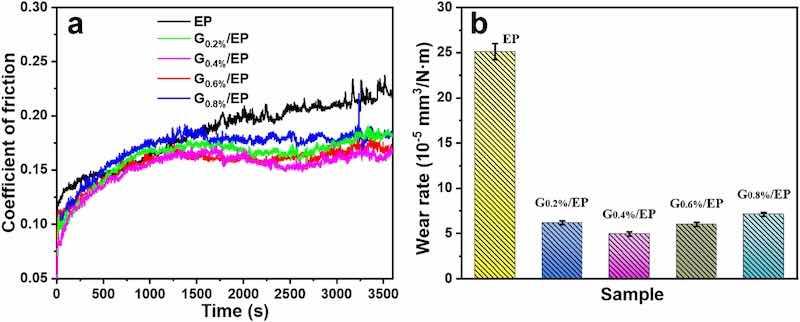
Fig. 4. Coefficient of friction curve (a) and average wear rates (b) of the samples.
In order to understand the wear mechanism of epoxy powder coating in depth, the OM, SEM surface morphologies and 3D profiles of the wear tracks of the samples were shown in Fig. 5 and Fig. 6, respectively. It can be distinctly observed that the EP coating presented wide (about 900 μm) and deep (about 65 μm > the coating's thickness) wear tracks (Fig. 5, Fig. 6), parallel grooves and furrows appeared along the sliding direction, and a large amount of wear debris occurred on the surface of wear track. The EDS spectrum at the corresponding wear track confirmed that the major element is Fe in the wear area, implying that the coating had peeled off to expose the steel substrate in a large area and the wear resistance of the coating was low. After introducing 0.2 wt% G nanosheet, the wear scar width (about 650 μm) and depth (about 17 μm < the coating's thickness) of the G0.2%/EP composite coating were reduced, and the debris and cracks of the wear track under normal load were greatly decreased. Compared with other the G/EP composite coatings, it can be found that the G0.4%/EP composite coating demonstrated the narrowest (approximately 620 μm) and shallowest (about 12 μm < the coating's thickness) wear tracks, suggesting that mild G nanoplate addition can remarkably improve the friction-wear resistance of epoxy powder coating. This was potentially due to the loose structure, poor adhesion strength and lack of lubricating phase of the EP sample, which makes it easy to peel off and delaminate during the rub process, ultimately leading to failure. The introduce of mild G sheet notably improved the compactness and adhesion properties of the coating, so that the coating was difficult to exfoliation and failure. In addition, the G nanosheets could effectively act as a self-lubricating matrix [20,27]. During the continuous friction test, the continuously exposed G form a lubricating film on the surface of the wear track, enhancing the tribological performance of the powder coating.
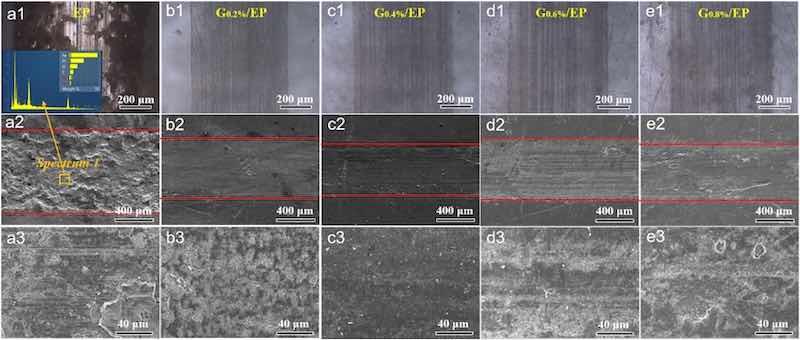
Fig. 5. Optical images and SEM micrography of wear tracks of the (a1-a3) EP, (b1-b3) G0.2%/EP, (c1-c3) G0.4%/EP, (d1-d3) G0.6%/EP and (e1-e3) G0.8%/EP coatings.

Fig. 6. The 3D profiles (a–e) and depth (f) of wear tracks of the samples.
3.4. Anti-corrosion performance
The electrochemical impedance spectroscopy (EIS) technology was employed to evaluate the protective behavior of the EP, and G/EP composite coatings during immersion in 3.5% NaCl solution, as shown in Fig. 7. In Nyquist plots, the larger the capacitive arc radius, the better the protective capacity of the sample [29]. In the case of the EP and G/EP samples, with the extension of immersion period, the capacitive arc radius presents a shrinking trend, indicating that the anti-corrosion ability of the coatings gradually decreases. This was potentially due to the gradually diffusion and infiltrate of corrosive medium in the coating, which increases the number of pores and defects, resulting in a decline in corrosion resistance [29]. Furthermore, the lowest frequency impedance modulus value (|Z|0.01 Hz) in Bode plots can effectively reflect the shielding performance of organic coating [29]. Obviously, the |Z|0.01 Hz value of the EP sample dropped from 2.38 × 106 Ω cm2 (1 d) to 1.26 × 104 Ω cm2 (50 d), implying the low corrosion resistance. Meanwhile, the |Z|0.01 Hz values of the G0.4%/EP and G0.6%/EP composite coatings were as high as 4.17 × 107 Ω cm2 and 3.09 × 107 Ω cm2 at the initial stage of immersion, which were both higher than that of the EP sample, hinting the enhancement of anti-corrosion performance. After immersing for 50 days, the |Z|0.01 Hz value of the G0.4%/EP sample still maintains a high level (9.65 × 105 Ω cm2), revealing the high corrosion protection capacity.
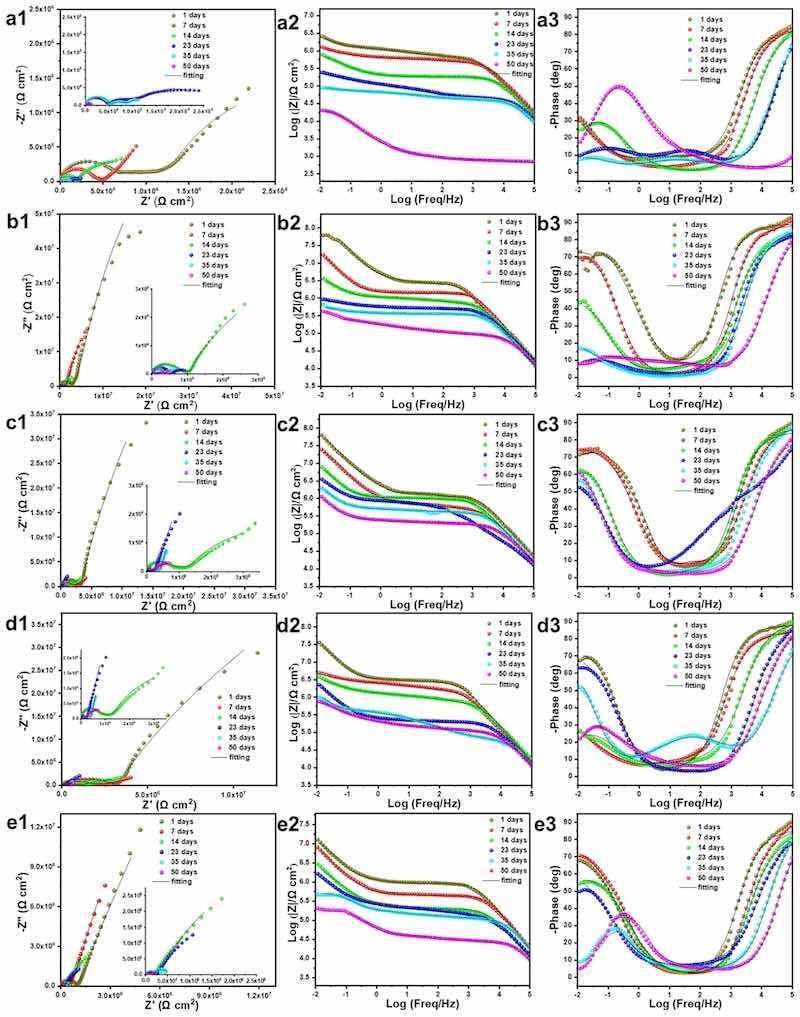
Fig. 7. Nyquist and bode plots of the (a1-c1) neat EP, G0.2%/EP, G0.4%/EP, G0.6%/EP, G0.8%/EP coatings immersed in 3.5% NaCl solution for different period.
The ZsimpWin software is further used to perform equivalent circuit fitting and analysis on the EIS data of the coatings at various immersion times, the results were shown in Fig. 8(a and b). In the equivalent circuit model, the Rs, Qc, Rc, Qdl, Rct respectively represented the solution resistance, coating capacitance, coating resistance, double layer capacitance and charge transfer resistance in the experiment system, the Zw represented the Warburg impedance related to the diffusion behavior of the composite coating. The variation of the Rc and Rct values of the EP and G/EP composite powder coatings with different immersion period were shown in Fig. 8(b and c). Rc is usually employed to assess the physical barrier effect of coating. Rct reflects the severity of the corrosion reaction on surface of metal substrate. The higher the values of Rc and Rct, the stronger the shielding effect and corrosion resistance of the coating [37,38]. It can be found that the Rc and Rct values of the powder coatings present a downward trend. During the entire immersion cycle, the Rc value of the EP sample was the lowest, and the Rc value of the G0.4%/EP sample always remained the highest, indicating that the addition of 0.4 wt% G nanosheet enhanced the tortuosity of penetration of aggressive media and water in the powder coating, demonstrating a labyrinth effect. In addition, the Rct values of the powder coatings showed a significant decline period of 7 d (EP), 14 d (G0.2%/EP), 23 d (G0.4%/EP), 14 d (G0.6%/EP), and 14 d (G0.8%/EP), suggesting the beginning of the corrosion reaction of the steel substrate. This reveals that the epoxy powder coating with 0.4 wt% G could effectively prevent the penetration of corrosive media and protect the steel substrate.
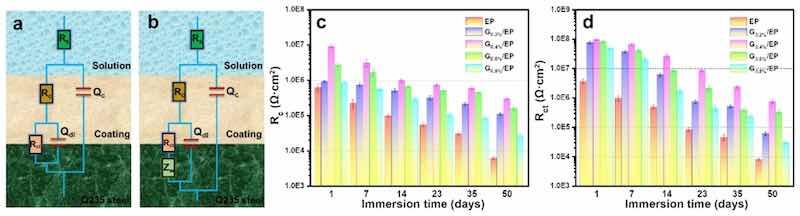
Fig. 8. (a–b) Equivalent electrical circuit model, Tendency of the (c) Rc and (d) Rct values of the EP and G/EP composite coatings immersed in 3.5% NaCl solution.
Fig. 9 showed the potentiodynamic polarization curve of the as-prepared samples immersed in 3.5% NaCl solution for 50 days. The corresponding electrochemical corrosion parameters were obtained by Tafel extrapolation method, as shown in Table 1. Compared with the EP powder coating, the epoxy powder coating with the addition of 0.2 wt% G could evidently positive shift the corrosion potential from −0.78 to −0.62 VS. SCE/V and reduce the corrosion current density from 4.11 × 10−6 to 7.26 × 10−8 A cm−2. The G0.4%/EP epoxy composite powder coating present the lowest corrosion current density (8.08 × 10−9 A cm−2) and the highest corrosion potential (−0.49 VS. SCE/V), suggesting the superior barrier properties. Compared with the EP coating, the corrosion current density of the G0.4%/EP coating has dropped by two orders of magnitude, and the corrosion potential has increased by 37.2%. Similar results were also observed in other literature [26].
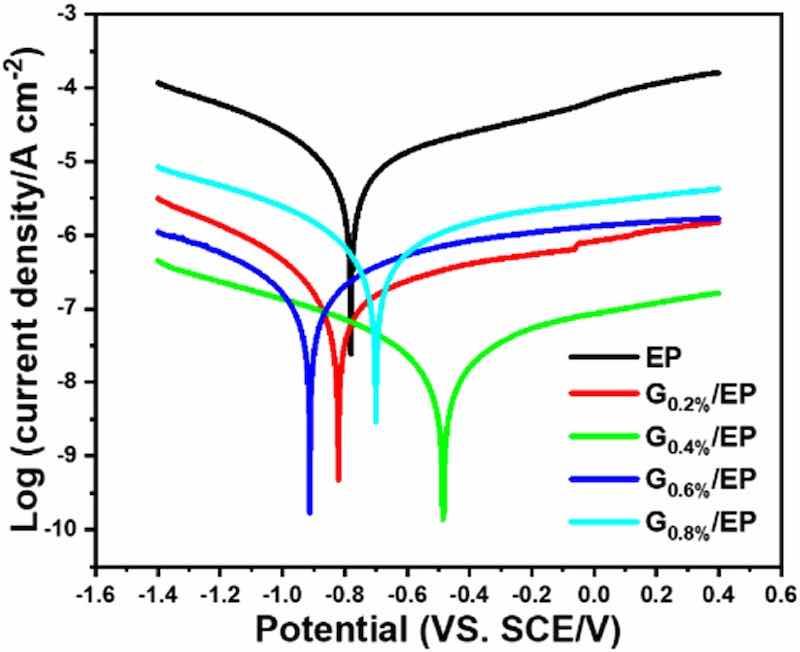
Fig. 9. Potentiodynamic polarization curve of the neat EP and G/EP composite coatings.
Table 1. Corrosion parameters of the samples.
| Sample | EP | G0.2%/EP | G0.4%/EP | G0.6%/EP | G0.8%/EP |
| Icorr (A·cm−2) | 4.11 × 10−6 | 7.26 × 10−8 | 8.08 × 10−9 | 7.96 × 10−8 | 2.75 × 10−7 |
| Ecorr (VS. SCE/V) | −0.78 | −0.62 | −0.49 | −0.71 | −0.70 |
Salt spray test (SST) can more intuitively observe the corrosion protection performance of the sample and be applied to assess the variation in the anti-corrosion behavior of the coating under various service periods [18]. Fig. 10 showed the boundary SST results of the EP and G/EP composite coatings. It can be clearly observed that after being placed in salt spray box for 72 h, the EP powder coating was severely rusted and damaged at the scratches, showing poor anti-corrosion behavior. However, no obvious rust spots were found in the G/EP composite powder coating. After 144 h SST, the dark yellow rust spots on the scratches of the EP powder coating increased greatly, the surface damage deteriorated and there was many corrosive pitting on the coating's surface. With respect to the samples with 0.2 wt% and 0.8 wt% G nanosheet, the rusting on the scratches on the sample's surface became evident, implying the corrosion resistance decreases. In the case of the G0.4%/EP sample, there was no apparent change in the surface of coating, hinting that the anti-corrosion capacity was maintained at a high class. After SST for 196 h, the rust on the scratches of the EP powder coating was heavily accumulated and spread to the surrounding region, causing serious damage and further degradation of the anti-corrosion ability. Interestingly, the rust of the G0.4%/EP composite powder coating was still hold at a slight level, suggesting that the sample exhibited an excellent anti-corrosion performance.
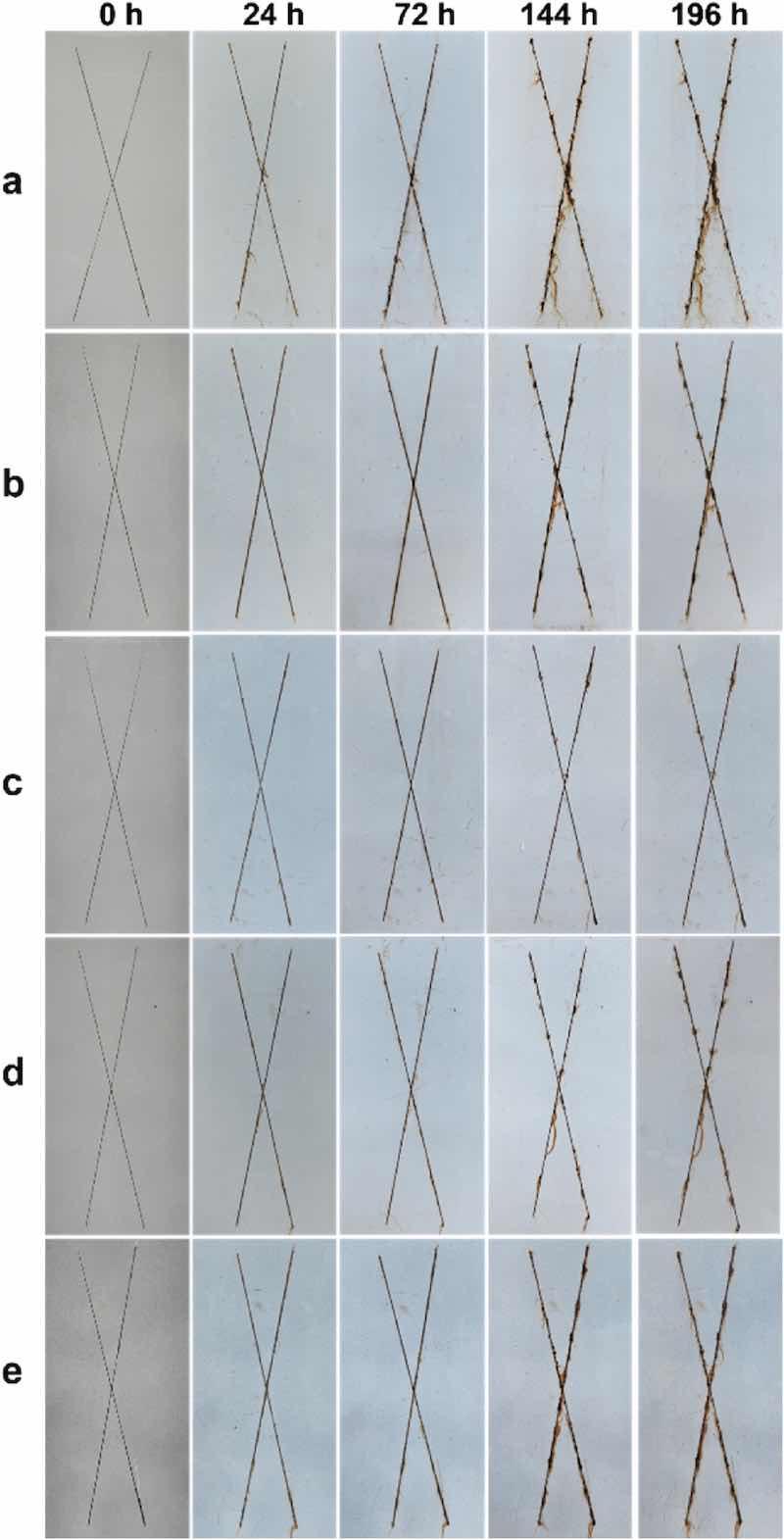
Fig. 10. Digital images of the salt spray test for the (a) EP, (b) G0.2%/EP, (c) G0.4%/EP, (d) G0.6%/EP, and (e) G0.8%/EP coating, respectively.
Combined with the relevant literature and our work, it is found that the lower nanoparticles content will improve the performance of the powder coating to a limited extent, and the high nanoparticles content will cause agglomeration and reduce the performance of powder coating. The physical properties, wear resistance and anti-corrosion behavior of powder coatings can be enhanced to some extent by embedding different kinds of nanoparticles with the best concentration. Fernández-Álvarez et al. found that the epoxy resin modified by 0.75 wt% hydrophilic SiO2 nanoparticles own the best wear resistance and mechanical properties [8]. Huttunen-Saarivirta et al. found that 0.5 and 1.0 wt.% montmorillonite nanoparticles slightly improved the tensile strength, ductility, and corrosion resistance of epoxy powder coatings [10]. Durand et al. revealed that composites containing 20 vol% ceramic particles had more excellent wear resistance. Fernández-Álvarez et al. found that the addition of 0.75–1% hydrophilia and 0.75% hydrophobic silica nanoparticles by ball milling improved the mechanical properties and wear resistance of epoxy powder coatings after UV irradiation [5]. Golgoon et al. found that inclusion of 10 wt% alumina nanoparticles improved the corrosion and wear resistance of polyester powder coatings [39]. Golgoon et al. reported that 1% ZnO nanoparticles could enhance the corrosion resistance of polyester powder coatings [40]. Golgoon et al. also reviewed studies on enhancing thermal properties, stability, optical clarity, mechanical properties, and corrosion and wear properties of powder coatings by adding clay, SiO2, TiO2, and Al2O3 nanoparticles [41]. Chen et al. reported that the tensile strength of nanocomposite coatings could be enhanced by loading 0.2 wt% m-MWCNTs into epoxy powder coatings, resulting in enhanced coating adhesion strength, tensile strength, and corrosion resistance [2]. Yu et al. found that the addition of 5 wt% calcium carbonate nanoparticles could enhance the impact resistance and cupping resistance of epoxy powder coating [14].
3.5. Mechanism of anti-wear and corrosion protection
Based on the above-mentioned analysis, it was easy to reveal that the G/EP composite powder coating present outstanding anti-wear and anti-corrosion performance. Fig. 11 showed the protective mechanism model of the coating. When the coatings sliding with the counterparts in periodic friction, the coating undergoes plastic deformation and partially cracking [20]. Part of the debris was mechanically compacted and adsorbed on the sliding surface under a normal load, and other debris was transferred to the edge of the wear track and accumulate to form a gully. Some surface defects of the coatings such as pinholes, voids and microcracks may extend below the sliding surface, and the extension of the defects may form perforated holes that provide channels for the penetration of corrosive media. After continuous reciprocating frictions, the diffusion of erosive media into the defect accelerates the damage and the coating peeling off, causing the coating to separate from the steel substrate, and ultimately defense function failure [27]. The loose microstructure, inferior adhesion and lack of lubricating phase in the EP powder coating make it easy to delaminate or peel off during the rub process, which will lead to the failure of the coating. After adding 0.4 wt% G nanosheets, the dense and adhesion properties of the G/EP composite coating were significantly enhanced. The high compactness and the coating-substrate adhesion strength were beneficial to suppress the deformation and microcracks of the coating and unfavorable to the spalling and delamination of the coating during the friction process, showing a shallow and narrow wear track. At the same time, the high specific surface area and hard G nanosheets were easy to form a lubricating transfer film on the wear surface, which improves the lubricating effect of the sliding contact interface [27]. In terms of corrosion resistance, the introduction of G sheet with good dispersibility could increase the tightening of the coating and reduce the penetration and diffusion paths of corrosive media. On the other hand, the impenetrability of G could complicate the infiltration path and effectively slow or prevent the corrosive solutions reaching to the steel substrate. Therefore, G0.4%/EP composite coating showed the excellent wear resistance and corrosion protection behavior.
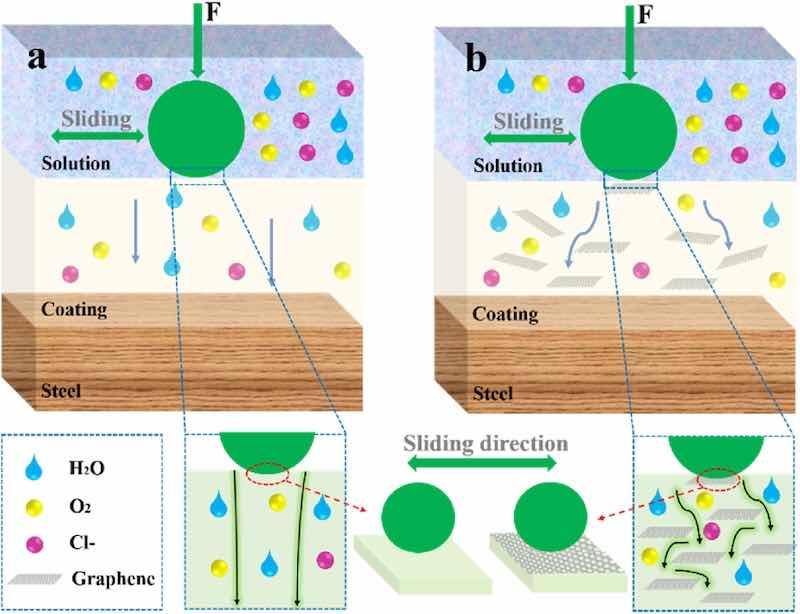
Fig. 11. Schematic diagram for understand the improvement mechanism of wear and corrosion resistance of the G/EP composite powder coating.
4. Conclusion
In this work, the EP, and G/EP composite powder coating were successfully prepared on carbon steel. The effects of G nanosheet concentration on microstructure, adhesion performance, tribological property and corrosion resistant of the organic epoxy powder coating were systematically studied. The main results were summarized as follows. Compared with the pure EP powder coating, the G/EP composite powder coatings with mild G addition possessed denser and almost defect-free microstructure. The G/EP composite powder coatings with 0.2–0.6 wt% G nanosheet displayed the superior coating-substrate adhesion behavior and impact resistant. In addition, the sliding friction test results showed the mean coefficient of friction and the wear rate of G/EP composite powder coating containing 0.4 wt% G were respectively decreased by 23.8 and 80.29% at most compared with the EP coating. After 50 days of immersion, the impedance modulus value of the G/EP coating remained at 9.65 × 105 Ω cm2, which was higher than that of the EP coating of 1.26 × 104 Ω cm2. In summary, the G/EP composite powder coatings introduced into 0.4 wt% G sheet demonstrated the outstanding comprehensive properties, combining adhesion, tribology and anti-corrosion performances, suggesting that the coating was an effective strategy to enhance the protection functional in rigorous mechanical-corrosion coupled environments.
Written by Jingwen Zhanga, Gang Konga, Shuao Lia, Yongpeng Lea, Chunshan Chea, Shuanghong Zhanga, Delin Laia, and Xinli Liaob
- A: School of Materials Science and Engineering, South China University of Technology, Guangzhou 510641, PR China
- B: Wenzhou Taichang Iron Tower Manufacturing Co., LTD, WenZhou 325000, PR China
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: The authors are thankful for the financial support of the Guangzhou Science and Technology Project (Grant No. 202102080408).
References
[1] Chen Q, Zhao Y-J, Teng J-L, Xia Y-Q, Yu B-Q, Zheng Y. Preparation and comparison anticorrosive properties of graphene anticorrosive coatings. IOP Conf Ser Mater Sci Eng 2020;774(1):012054.
[2] Chen S, Wang X, Zhu G, Lu Z, Zhang Y, Zhao X, et al. Developing multi-wall carbon nanotubes/Fusion-bonded epoxy powder nanocomposite coatings with superior anti- corrosion and mechanical properties. Colloids Surf A Physicochem Eng Asp 2021;628:127309.
[3] Chowaniec A, Czarnecki S, Sadowski Ł. The effect of the amount and particle size of the waste quartz powder on the adhesive properties of epoxy resin coatings. Int J Adhesion Adhes 2021;117:103009.
[4] Kargarfard N, Simon F, Schlenstedt K, Ulischberger L, Voit B, Gedan-Smolka M, et al. Self-stratifying powder coatings based on eco-friendly, solvent-free epoxy/silicone technology for simultaneous corrosion and weather protection. Prog Org Coating 2021;161:106443.
[5] Fernandez-Alvarez M, Velasco F, Bautista A. Performance of ultraviolet exposed epoxy powder coatings functionalized with silica by hot mixing. J Mater Res Technol 2021;10:1042e57.
[6] Kugler S, Ossowicz-Rupniewska P, Wierzbicka E, Łopinski J. Anhydride-cured epoxy powder coatings from natural-origin resins. Hardeners, and Fillers, Coatings 2021;5:11.
[7] Ramezanzadeh M, Sanaei Z, Ramezanzadeh B. The influence of steel surface treatment by a novel eco-friendly praseodymium oxide nanofilm on the adhesion and corrosion protection properties of a fusion-bonded epoxy powder coating. J Ind Eng Chem 2018;62:427e35.
[8] Fernandez-Alvarez M, Velasco F, Bautista A, Galiana B. Functionalizing organic powder coatings with nanoparticles through ball milling for wear applications. Appl Surf Sci 2020;513:145834.
[9] Fernandez-Alvarez M, Velasco F, Torres-Carrasco M, Bautista A. Hindering the decrease in wear resistance of UV- exposed epoxy powder coatings by adding nano-SiO2 through ball milling. Wear 2021:480e1. 203935.
[10] Huttunen-Saarivirta E, Vaganov GV, Yudin VE, Vuorinen J. Characterization and corrosion protection properties of epoxy powder coatings containing nanoclays. Prog Org Coating 2013;76(4):757e67.
[11] Luo S, Zheng Y, Li J, Ke W. Effect of curing degree and fillers on slurry erosion behavior of fusion-bonded epoxy powder coatings. Wear 2003;254(3e4):292e7.
[12] Sharifi M, Ebrahimi M, Jafarifard S. Preparation and characterization of a high performance powder coating based on epoxy/clay nanocomposite. Prog Org Coating 2017;106:69e76.
[13] Fernandez-Alvarez M, Velasco F, Bautista A. Epoxy powder coatings hot mixed with nanoparticles to improve their abrasive wear. Wear 2020:448e9. 203211.
[14] Yu H, Wang L, Shi Q, Jiang S, Jiang G. Preparation of epoxy resin/CaCO3 nanocomposites and performance of resultant powder coatings. J Appl Polym Sci 2010;101(4):2656e60.
[15] Kalaee M, Akhlaghi S, Nouri A, Mazinani S, Mortezaei M, Afshari M, et al. Effect of nano-sized calcium carbonate on cure kinetics and properties of polyester/epoxy blend powder coatings. Prog Org Coating 2011;71(2):173e80.
[16] Mirabedini SM, Kiamanesh A. The effect of micro and nano- sized particles on mechanical and adhesion properties of a clear polyester powder coating. Prog Org Coating 2013;76(11):1625e32.
[17] Shen L, Li Y, Zhao W, Miao L, Xie W, Lu H, et al. Corrosion protection of graphene-modified zinc-rich epoxy coatings in dilute NaCl solution. ACS Appl Nano Mater 2018;2(1):180e90.
[18] Wu Y, Jiang F, Qiang Y, Zhao W. Synthesizing a novel fluorinated reduced graphene oxide-CeO2 hybrid nanofiller to achieve highly corrosion protection for waterborne epoxy coatings. Carbon 2021;176:39e51. 4160 j o u r n a l o f m a t e r i a l s r e s e a r c h a n d t e c h n o l o g y 2 0 2 2 ; 2 0 : 4 1 4 8e4 1 6 0 [19] Zhu X, Zhao H, Wang L, Xue Q. Bioinspired ultrathin graphene nanosheets sandwiched between epoxy layers for high performance of anticorrosion coatings. Chem Eng J 2020;410(7):128301.
[20] Ye Y, Zhang D, Liu T, Liu Z, Pu J, Liu W, et al. Superior corrosion resistance and self-healable epoxy coating pigmented with silanizied trianiline-intercalated graphene. Carbon 2019;142:164e76.
[21] Cui G, Bi Z, Zhang R, Liu J, Yu X, Li Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem Eng J 2019;373:104e21.
[22] Liu S, Gu L, Zhao H, Chen J, Yu H. Corrosion resistance of graphene-reinforced waterborne epoxy coatings. J Mater Sci Technol 2016;32(5):425e31.
[23] Zhang Z, Zhang W, Li D, Sun Y, Wang Z, Hou C, et al. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. Int J Mol Sci 2015;16(1):2239e51.
[24] Campo M, Jimenez-Suarez A, Ure~ na A. Tribological properties of different types of graphene nanoplatelets as additives for the epoxy resin. Appl Sci 2020;10(2):4363.
[25] Zhang Y, Zhang D, Wei X, Zhong S, Wang J. Enhanced tribological properties of polymer composite coating containing graphene at room and elevated temperatures. Coatings 2018;8(3):91.
[26] Li X, Wang F, Mao J. Preparation and properties of thermosetting powder/graphene oxide coatings for anticorrosion application. J Appl Polym Sci 2019;136(48):48264.
[27] Chen C, Qiu S, Cui M, Qin S, Yan G, Zhao H, et al. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017;114:356e66.
[28] Ye Y, Zhang D, Li J, Liu T, Pu J, Zhao H, et al. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti- corrosion and anti-wear fields. Corrosion Sci 2019;147:9e21.
[29] Wu Y, Zhao W, Lu Z, Wang L. Fluorinated graphene film for corrosion control on copper: experimental and theoretical studies. Carbon 2021;179:445e57.
[30] Qiu S, Liu G, Li W, Zhao H, Wang L. Noncovalent exfoliation of graphene and its multifunctional composite coating with enhanced anticorrosion and tribological performance. J Alloys Compd 2018;47:60e70.
[31] Zhang J, Zhou S, Wang Y, Wang Y, Wang C, Lu X, et al. Enhancing anti-corrosion and antifouling properties of Cu/ GLC composite film for marine application. Surf Coating Technol 2019;375:414e26.
[32] Davim JP. Tribology for engineers. 2011.
[33] Davim JP. Mechanical and industrial engineering. Springer Berlin Heidelberg; 2022.
[34] Davim JP. Modern mechanical engineering. Springer Berlin Heidelberg; 2014.
[35] Davim JP. Nanocomposites 2011.
[36] Annappa AR, Basavarajappa S, Davim JP. Effect of organoclays on mechanical properties of glass fiber- reinforced epoxy nanocomposite. Polym Bull 2022;79:5085e103.
[37] Zhou S, Wu Y, Zhao W, Yu J, Jiang F, Wu Y, et al. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater Des 2019;169:107694.
[38] Mirzakhanzadeh Z, Kosari A, Moayed MH, Naderi R, Taheri P, Mol JMC. Enhanced corrosion protection of mild steel by the synergetic effect of zinc aluminum polyphosphate and 2-mercaptobenzimidazole inhibitors incorporated in epoxy-polyamide coatings. Corrosion Sci 2018;138:372e9.
[39] Golgoon A, Aliofkhazraei M, Toorani M, Moradi MH, Sabour Rouhaghdam A. Corrosion and wear behavior of alumina- polyester nanocomposite coatings. Polym Eng Sci 2017;57:846e56.
[40] Golgoon A, Aliofkhazraei M, Toorani M, Moradi MH, Sabour Rouhaghdam A, Asgari M. Corrosion behavior of ZnO- polyester nanocomposite powder coating. Anti-corrosion Methods & Mater 2017;64:380e8.
[41] Golgoon A, Aliofkhazrae M, Toorani M. Nanocomposite protective coatings fabricated by electrostatic spray method. Protect Met Phys Chem Surface 2018;54:192e221.



































