Anodized aluminum has the potential to save thousands of lives through personalized healthcare. Precision or personalized medicine is health care tailored to the individual and there is a growing need for inexpensive diagnostics in this field.
A diagnostic based on anodized aluminum is being developed to assess the health of an individual by visualizing a color change. A thin film of aluminum is deposited onto a tantalum coated substrate and then anodized to generate interference colors. Control of the optical properties of the nanoporous aluminum oxide can be accomplished by manipulating the pore size during anodization through the voltage and electrolyte type.
We demonstrate the ability to detect color shifts from molecules as small as 2nm at picomolar (10-12) concentrations on the anodized surface.
The following paper was presented at the 2017 Anodizing Conference at Aluminum Anodizing & Extrusion Summit in Westminster, Colorado, by Matthew Nickel, University of Alberta; Hillary Sweet, University of Alberta; and Robert Burrell, Prominent Medical Inc.
I. Precision Medicine
 Matthew NickelPrecision or personalized medicine is healthcare tailored to the individual. Up until recently western medicine has worked under the assumption that diseases progress at about the same rate in most individuals, showing similar symptoms at similar times and treated in similar ways. This has worked for most forms of disease in the past, however it is a poor strategy for cancer treatment. Cancer is intrinsically many different types of diseases due to the nature by which it comes about: genetic mutation to form cells that multiply uncontrollably. Healthy cells have check stops that signal when to grow and when not to. Cancer cells, on the other hand, have had a series of genetic mutations that destroy or bypass these check stops and therefore the cell just continues to grow and divide, developing into a tumor. These genetic mutations are also all very different. In fact, there are over 100 different types of cancer with new types being discovered every year [1]. Therefore, a blanket treatment does not exist and we must instead develop different treatments and diagnostics for each type of cancer. This is one of the main drivers behind the concept of precision medicine.
Matthew NickelPrecision or personalized medicine is healthcare tailored to the individual. Up until recently western medicine has worked under the assumption that diseases progress at about the same rate in most individuals, showing similar symptoms at similar times and treated in similar ways. This has worked for most forms of disease in the past, however it is a poor strategy for cancer treatment. Cancer is intrinsically many different types of diseases due to the nature by which it comes about: genetic mutation to form cells that multiply uncontrollably. Healthy cells have check stops that signal when to grow and when not to. Cancer cells, on the other hand, have had a series of genetic mutations that destroy or bypass these check stops and therefore the cell just continues to grow and divide, developing into a tumor. These genetic mutations are also all very different. In fact, there are over 100 different types of cancer with new types being discovered every year [1]. Therefore, a blanket treatment does not exist and we must instead develop different treatments and diagnostics for each type of cancer. This is one of the main drivers behind the concept of precision medicine.
 Hillary SweetA perfect example of this is in papillary thyroid cancer (PTC). PTC is the most common type of thyroid cancer, accounting for over 80% of all cases [2]. Of these cases, 60% will metastasize to the lymph nodes and are determined to be of higher risk [3]. Recent work has shown that patients with tumors that exhibit a special protein biomarker called PDGFRα will be 3 times more likely to have nodal metastases and 18 times more likely to have a recurrence within 5 years compared to patients without this biomarker [4]. All patients currently undergo the same highly invasive treatment whether or not their tumor presents PDGFRα, and here is the importance of personalized medicine. There is no technology today that can be used in the operating room to quickly diagnose (<20 minutes) the presence of this molecule. Current techniques involve a day in the lab of a central laboratory; a wait time that’s not possible for a patient open and on the operating table. Because of this, surgeons currently do worst-case scenario treatment on all patients, involving total removal of the thyroid, potential loss of nerves, and extensive dissection of the lymph nodes up and down the throat [5]. If the surgeon knew the cancer was benign, a much less invasive surgery would suffice. This means that the 40% of patients without the metastatic-linked biomarker go through an invasive and unnecessary treatment, resulting in increased suffering where none is needed. This is just one case where precision medicine would really make a difference.
Hillary SweetA perfect example of this is in papillary thyroid cancer (PTC). PTC is the most common type of thyroid cancer, accounting for over 80% of all cases [2]. Of these cases, 60% will metastasize to the lymph nodes and are determined to be of higher risk [3]. Recent work has shown that patients with tumors that exhibit a special protein biomarker called PDGFRα will be 3 times more likely to have nodal metastases and 18 times more likely to have a recurrence within 5 years compared to patients without this biomarker [4]. All patients currently undergo the same highly invasive treatment whether or not their tumor presents PDGFRα, and here is the importance of personalized medicine. There is no technology today that can be used in the operating room to quickly diagnose (<20 minutes) the presence of this molecule. Current techniques involve a day in the lab of a central laboratory; a wait time that’s not possible for a patient open and on the operating table. Because of this, surgeons currently do worst-case scenario treatment on all patients, involving total removal of the thyroid, potential loss of nerves, and extensive dissection of the lymph nodes up and down the throat [5]. If the surgeon knew the cancer was benign, a much less invasive surgery would suffice. This means that the 40% of patients without the metastatic-linked biomarker go through an invasive and unnecessary treatment, resulting in increased suffering where none is needed. This is just one case where precision medicine would really make a difference.
 Robert BurrellSo we know that precision medicine is useful and can help save the suffering of many people, but how do we implement it? Up until recently, almost all testing of patient samples, be it blood, urine, or tissue, was conducted in large central laboratories with highly trained staff and large sophisticated equipment. This is still largely the case, however, in the last few years there has been a huge movement towards what’s called point-of-care testing (POCT). The idea behind POCT has mainly been to miniaturize the big, bulky lab equipment down to the size of a phone or desktop computer and automate it. This is seen with emerging products such as the mChip or i-STAT where the current standard diagnostic technologies, PCR (polymerase chain reaction) and ELISA (enzyme linked immunosorbent assay), are made to function in a portable benchtop or handheld device, Figure 1 [6, 7]. The main issues with these technologies has been the complexity by which they operate, leading to increased costs and difficulty in mass manufacture [7].
Robert BurrellSo we know that precision medicine is useful and can help save the suffering of many people, but how do we implement it? Up until recently, almost all testing of patient samples, be it blood, urine, or tissue, was conducted in large central laboratories with highly trained staff and large sophisticated equipment. This is still largely the case, however, in the last few years there has been a huge movement towards what’s called point-of-care testing (POCT). The idea behind POCT has mainly been to miniaturize the big, bulky lab equipment down to the size of a phone or desktop computer and automate it. This is seen with emerging products such as the mChip or i-STAT where the current standard diagnostic technologies, PCR (polymerase chain reaction) and ELISA (enzyme linked immunosorbent assay), are made to function in a portable benchtop or handheld device, Figure 1 [6, 7]. The main issues with these technologies has been the complexity by which they operate, leading to increased costs and difficulty in mass manufacture [7].
![Figure 1. Left: mChip point-of-care diagnostic developed by Claros Diagnostics Inc. [6]. Right: i-STAT handheld diagnostic developed by Abbott point-of-care diagnostics [34].](/images/images/acc_nickelsweetburrell/1.jpg)
The most commonly known type of on-site diagnostic is the lateral flow assay. This is the technology of the at-home pregnancy test and has been commercially available to the public for the last 30 years [8]. It is inexpensive, instrument free, and can be used essentially anywhere, without training. Where it is limited is in assay design flexibility and sensitivity. Most lateral flow assays can only process urine or saliva samples, and they tend to be single analyte tests, not able to detect multiple biomarkers or molecules. Although technologies like the mChip and i-STAT have improved on this, they are not nearly as affordable, with test strips costing $30-50 USD each and the handheld or benchtop readers being anywhere from $1,000-15,000 USD [9, 10]. The solution to this is in a fundamentally new approach to diagnostics.
II. Thin Film Diagnostics
If you have ever seen oil on water and noticed the rainbow of colors you have experienced thin film interference. The colors are a result of the difference in optical properties between the water and the oil. Light is reflected off the interface between the oil and air and the interface between the oil and water. These two reflections will recombine and interfere with one another to create a color, Figure 2. This color is dependent on the thickness of the oil layer and thus the rainbow of colors you see are a result of the different thicknesses of oil on water [11].
![Figure 2. Left: Thin film interference created by oil on water [11]. Right: A schematic of light interference that occurs from oil on water [33].](/images/images/acc_nickelsweetburrell/2.jpg)
Interference colors are also generated when certain types of metals (e.g. tantalum) are anodized at high voltages [12]. These colors are generated due to an oxide film on the surface of the metal, in a similar way that color is generated by oil on water. The oxide is semi- transparent and the film is thin enough to cause light reflected from the surface of the oxide and light reflected from the oxide-metal interface to interfere and generate different colors. The colors observed depend on the optical path length (OPL), which is the length of the film through which the light travels (d) multiplied by the refractive index of the material (η), as shown in equation (1) [13]. The refractive index is a material dependent constant that is equal to c/v, where c is the speed of light in a vacuum and v is the speed of light in the medium or material [14].
𝑂𝑃𝐿 = 𝜂𝑑 (1)
Thus, if there is a small change in the thickness of the film, a change in color can be seen due to the change in the optical path length.
In the field of thin film diagnostics, this color generating principle can be used to detect small molecules or proteins such as antibodies and antigens. Antibodies are blood proteins that are part of the immune system. Their main purpose is to bind to foreign substances (antigens) to alert the body to an invader. The basis of this type of diagnostic device is to immobilize an antibody on a thin film surface to generate a color. If a patient’s blood sample is placed on the surface of the device and contains the antigen associated with the immobilized antibody, then the antigen will bind and create a visible color change after washing and drying, due to the thickness increase of the film.
These types of diagnostics were first developed in the 1970’s by Ivar Giaever [15, 16]. Giaever recognized that thin film interference could be used to detect antibody-antigen reactions and developed two patents in ’73 and ’74 [17, 18]. However, he experienced a large problem with sensitivity and the time it took for the antibodies and antigens to react on the surface of the device. In attempts to increase sensitivity, another patent was filed in 1975 [19], however the device remained complex and required anywhere from 4 to 16 hours for results.
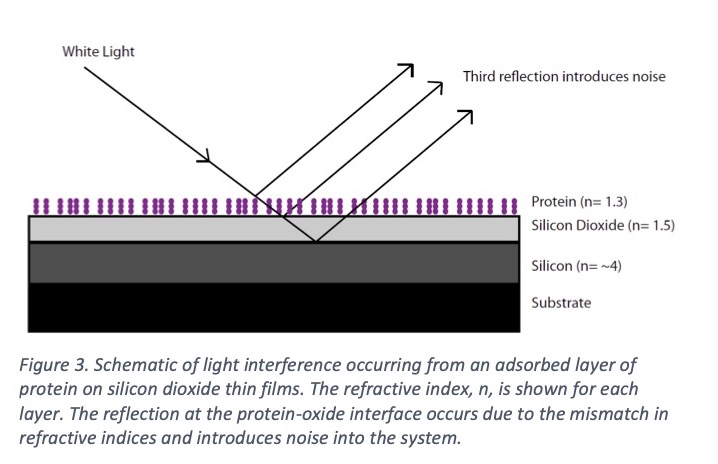
In 1983 Nygren et al. introduced a thin film device based on a silicon and a thin silicon dioxide layer [20, 21]. Molecules as small as 0.7nm could be seen clearly by a change of color of the slide [21, 22]. The problem was that the refractive index of the thin silicon dioxide layer could not be tailored. This limited the flexibility of the device as interference colors are most sensitive when the protein or molecular layers added are of a similar refractive index to their substrate. At each point where there is a change in refractive index, a reflection of light occurs. If the protein layers added to the surface do not have a similar refractive index to the oxide, a reflection occurs at the protein-oxide interface, Figure 3. This reflection introduces noise into the system and reduces the sensitivity of the device. What was required was a material where the refractive index could be tuned to match the substance you wanted to detect.
III. Anodized Aluminum for Thin Film Diagnostics
Recently there has been an increase of interesting and new applications for anodized aluminum. The ability to create a regular hexagonal array of pores with diameters tailored in the nanometers has led to various research in nanostructured materials and the use of porous anodic alumina in the field of medicine for biosensors [23, 24], molecular and ion separation [25], drug delivery devices [26], and biocompatible implants for improved tissue integration [27]. With over 3000 papers published on porous aluminum oxide in the last 10 years, there appears to be no end to the applications of this material (Web of Science, July 2017).
As stated before, the problem with previous thin film diagnostics was the lack of a material with a tunable refractive index. To fix this problem, Burrell et al. [28] patented a device to produce thin film interference colors based on anodized aluminum thin films.
 The device is comprised of a thin tantalum layer (200 nm) deposited onto a glass or polymer substrate, followed by a thin aluminum layer (90-150 nm) deposited onto the tantalum. When anodization is carried out, a porous aluminum oxide is produced and a thin tantalum oxide barrier layer, Figure 4. The tantalum is the color generating metal, with the color dependent on the thickness of the oxide layer. The porous aluminum oxide acts as the layer with an adjustable refractive index; by changing the electrolyte or voltage during anodization, the pore size and density can be changed which influences the percentage of air in the final film.
The device is comprised of a thin tantalum layer (200 nm) deposited onto a glass or polymer substrate, followed by a thin aluminum layer (90-150 nm) deposited onto the tantalum. When anodization is carried out, a porous aluminum oxide is produced and a thin tantalum oxide barrier layer, Figure 4. The tantalum is the color generating metal, with the color dependent on the thickness of the oxide layer. The porous aluminum oxide acts as the layer with an adjustable refractive index; by changing the electrolyte or voltage during anodization, the pore size and density can be changed which influences the percentage of air in the final film.
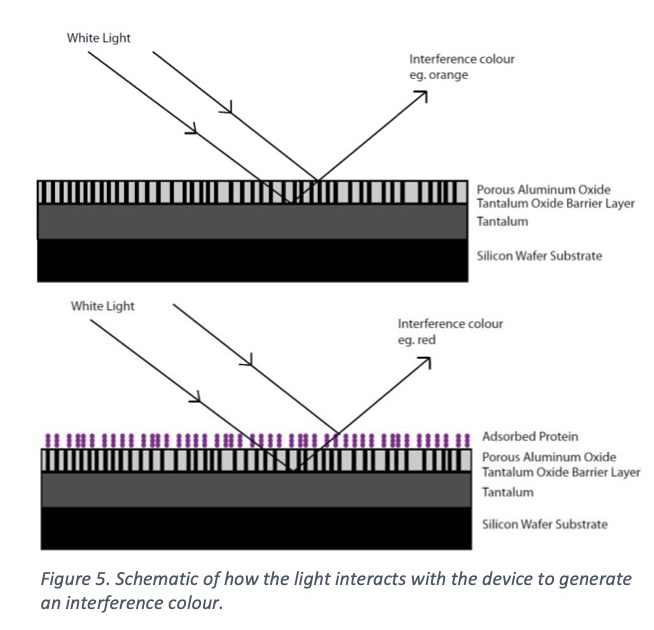
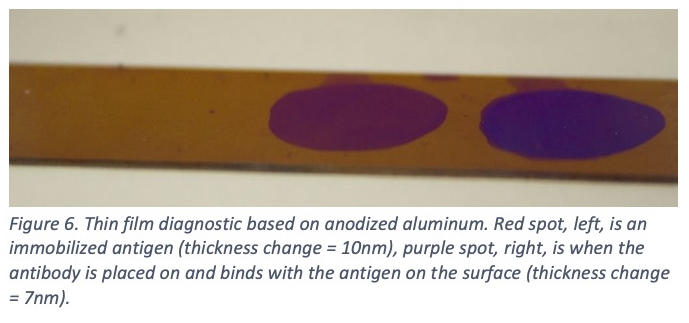
Air has a refractive index of 1, alumina has a refractive index of 1.7 [29], and protein has a refractive index of anywhere from 1.3-1.5 [30, 31]. Thus, the refractive index can be adjusted to match protein by changing the percentage of air in the aluminum oxide film. Figure 5 is a schematic of how the light interacts with the protein on the surface to change the interference color generated. The increased sensitivity brought about by tuning the refractive index allows for the most sensitive color shifts and even picomolar (10-12) surface concentrations can be observed very clearly. Figure 6 shows a colored spot where protein has been placed on the surface (left, red spot). When an antibody to that specific protein is then applied to the surface, it binds and creates a strong color shift in 15 minutes or less (right, purple spot).
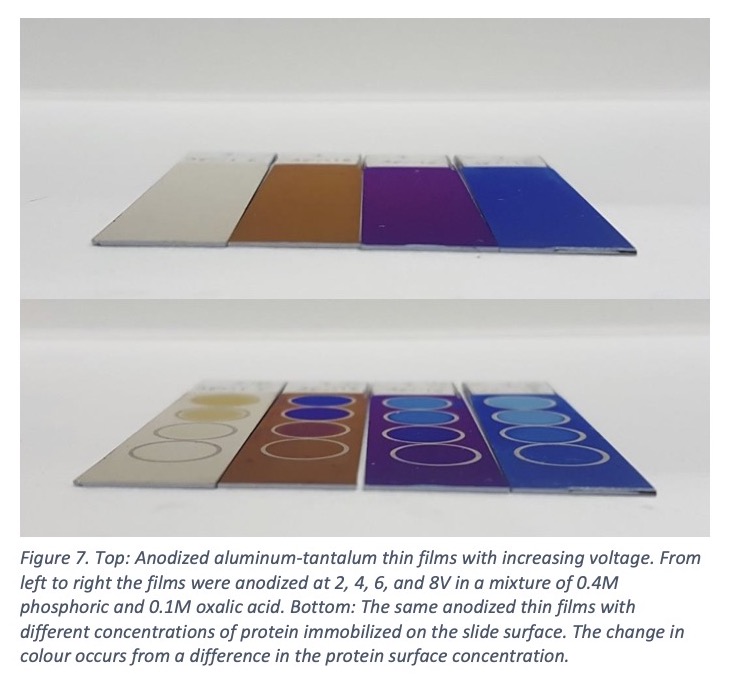
 Our lab works on the development and understanding of this device. The anodization process must be carefully carried out, as differences in the film thickness of 2 nm will generate a change in color. Both the pore size and density will shift the complex refractive index of the film and create color changes. Figure 7 (top) shows how the colors change by increasing the voltage.
Our lab works on the development and understanding of this device. The anodization process must be carefully carried out, as differences in the film thickness of 2 nm will generate a change in color. Both the pore size and density will shift the complex refractive index of the film and create color changes. Figure 7 (top) shows how the colors change by increasing the voltage.
Strong color shifts are observed when proteins or small molecules bind to the surface, Figure 7 (bottom). The simplicity of a dipstick-style test combined with the inexpensive materials used and the high sensitivity (picomolar concentrations) make thin film diagnostics based on anodized aluminum very attractive. It is also easy to imagine a test with many different proteins or antibodies attached to a small area of the surface. A small spot of blood could test for many types of diseases or biomarkers by simply checking for a color change in each area.
As we continue to develop this diagnostic, we have had to consider many aspects of the anodization process. These include: etching rate of the surface due to the electrolyte used, temperature of the anodization bath, and pore size and density with respect to the voltage used. The future technical challenges lie in mass anodization using a roll-to-roll process, however we believe this to be achievable as continuous anodizing lines have been done before in the canning and architectural industries [32].
IV. Conclusion
In the future, we imagine a world of democratized diagnostics. A world where any individual can purchase a diagnostic test at the drug store, place a sample of their blood on the test surface, and read the device by eye. Personalized healthcare is the direction forward for medicine, but we need a technology that can make it easy and affordable to anyone. We believe anodized aluminum is this technology and has the potential to save thousands of lives in the future.
Matthew Nickel 1; Hillary Sweet 1,2; Robert Burrell 1,2,3
- 1 Department of Chemical & Materials Engineering, University of Alberta, Edmonton, AB, Canada
- 2 Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada
- 3 Prominent Medical Inc., Edmonton, AB, Canada
Matthew Nickel, PhD: Matthew, founder of SN Biomedical, is a problem solver, who wakes up inspired every day to help build a better world. He graduated with a PhD in Materials Engineering and Biomedical Engineering from the University of Alberta in 2018, which specialized in nano-structured materials in medicine. Matthew's Bachelors degree was in Materials Engineering from the University of Alberta, and some of his hobbies include learning languages, playing the piano, and spending time with family.
Hillary Sweet, PhD: Hillary, founder of SN Biomedical, is a determined and hardworking individual, who strives to apply the skills she has acquired to help others. She graduated with a PhD in Materials Engineering and Biomedical Engineering from the University of Alberta in 2018, specializing in point-of-care clinical diagnostics. Hillary's Bachelors degree was in Mechanical Engineering from Queen's University, and in her downtime she enjoys playing sports, painting, and exploring new cuisine.
Robert E. Burrell, PhD: Dr. Burrell is currently a Canada Research Chair in Nanostructured Biomaterials and a Professor of Chemical and Materials Engineering in the Faculty of Engineering as well as Professor and Chair of Biomedical Engineering in the Faculty of Medicine and Dentistry at the University of Alberta. He holds a B.Sc.(Zoology) and M.Sc.(Microbial Toxicology) from Guelph and a Ph.D.(Toxicology) and Post Doctoral Fellowship (Chemical Engineering) from Waterloo. Dr. Burrell has established a program for the development of advanced nanomaterials for biomedical applications. He is one of the world’s leading authorities on the use of these materials for medical applications including 1) control of microbial growth on a wide range of devices and 2) control of the inflammatory response after injury. He invented and developed the world’s first commercially successful therapeutic application of nanotechnology – Acticoat wound dressings. He is a named inventor on more than 290 patents and pending patents worldwide.
References
[1] "What is Cancer?," National Cancer Institute, 9 February 2015. [Online].
[2] J. Zhang, P. Wang, M. Dykstra, P. Gelebart, D. Williams, R. Ingham, E. E. Adewuyi, R. Lai and T. McMullen, "Platelet-derived growth factor receptor-alpha promotes lymphatic metastases in papillary thyroid cancer," Journal of Pathology, vol. 228, no. 2, pp. 241-250, 2012.
[3] A. R. Shaha, J. P. Shah and T. R. Loree, "Patterns of Failure in Differentiated Carcinoma of the Thyroid based on Risk Groups," Journal of the Sciences and Specialties of the Head and Neck, pp. 26-30, 1998.
[4] A. Lopez-Campistrous, E. E. Adewuyi, M. G. K. Benesch, Y. M. Ko, R. Lai, A. Thiesen, J. Dewald, P. Wang, K. Chu, S. Ghosh, D. C. Williams, L. J. Vos, D. N. Brindley and T. P. McMullen, "PDGFR alpha Regulates Follicular Cell Differentiation Driving Treatment Resistance and Disease Recurrence in Papillary Thyroid Cancer," EBioMedicine, vol. 12, pp. 86-97, 2016.
[5] T. McMullen, Personal Communication.
[6] C. D. Chin, V. Linder and S. K. Sia, "Commercialization of microfluidic point-of-care diagnostic devices," Lab Chip, vol. 12, pp. 2118-2134, 2012.
[7] C. Dincer, R. Bruch, A. Kling, P. S. Dittrich and G. A. Urban, "Multiplexed Point-of-Care Testing - xPOCT," Trends in Biotechnology, pp. 1-15, 2017.
[8] T. R. Holford, F. Davis and S. P. Higson, "Recent trends in antibody based sensors," Biosensors and Bioelectronics, vol. 34, pp. 12-24, 2012.
[9] J. Hu, X. Cui, Y. Gong, X. Xu, B. Gao, T. Wen, T. Lu and F. Xu, "Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care," Biotechnology Advances, vol. 34, pp. 305-320, 2016.
[10] C. D. Chin, Y. K. Cheung, T. Laksanasopin, M. M. Modena, S. Y. Chin, A. A. Sridhara, D. Steinmiller, V. Linder, J. Mushingantahe, G. Umviligihozo, E. Karita, L. Mwambarangwe, S. L. Braunstein, J. van de Wijgert, R. Sahabo, J. E. Justman, W. El-Sadr and S. K. Sia, "Mobile Device for Disease Diagnosis and Data Tracking in Resource-Limited Settings,"
[11] R. Nave, "Oil Film Interference," Hyperphysics, [Online].
[12] A. Charlesby and J. J. Polling, "The Optical Properties of Thin Oxide Films on Tantalum," Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences, vol. 227, pp. 434-447, 1955.
[13] F. A. Jenkins and H. E. White, Fundamentals of Optics Third Edition, New York: McGraw- Hill Book Company, Inc., 1957.
[14] D. Halliday, R. Resnick and J. Walker, Fundamentals of Physics 9th Edition, Jefferson City: John Wiley & Sons, Inc., 2011.
[15] I. Giaever, "The antigen-antibody reaction: a visual observation," Journal of Immunology, vol. 110, pp. 1424-1426, 1973.
[16] I. Giaever and R. J. Laffin, "Visual detection of hepatitis B antigen," Proceedings of the National Academy of Science USA, vol. 71, pp. 4533-4535, 1974.
[17] I. Giaever, "Method and apparatus for immunological detection of biological particles". United States of America Patent 3,853,467, 10 December 1974.
[18] I. Giaever, "Substrate for immunological tests and method of fabrication thereof". US Patent 3,926,564, 16 December 1975.
[19] I. Giaever , "Diagnostic device for visually detecting presence of biological particles". US Patent 3,979,184, 7 September 1976.
[20] B. H. Nygren, T. Sandström, J. E. Stenberg and L. B. Stilbert, "Method and member for detecting and/or measuring the concentration of a chemical substance". US Patent 4,558,012, 10 December 1985.
[21] H. Nygren, T. Sandström and M. Stenberg, "Direct Visual Detection of Protein Antigen: Importance of Surface Concentration," Journal of Immunological Methods, vol. 59, pp. 145-149, 1983.
[22] T. Sandström, M. Stenberg and H. Nygren, "Visual detection of organic monomolecular films by interference colors," Applied Optics, vol. 24, no. 4, pp. 472-479, 1985.
[23] S. D. Alvarez, C. Li, C. E. Chiang, I. K. Schuller and M. J. Sailor, "A Label-Free Porous Alumina Interferometric Immunosensor," ACS Nano, vol. 3, no. 10, pp. 3301-3307, 2009.
[24] S. Pan and L. J. Rothberg, "Interferometric Sensing of Biomolecular Binding Using Nanoporous Aluminum Oxide Templates," Nano Letters, vol. 3, no. 6, pp. 811-814, 2003.
[25] H. U. Osmanbeyoglu, T. B. Hur and H. K. Kim, "Thin alumina nanoporous membranes for similar size biomolecule separation," Journal of Membrane Science, vol. 343, no. 1-2, pp. 1-6, 2009.
[26] S. Simovic, D. Losic and K. Vasilev, "Controlled drug release from porous materials by plasma polymer deposition," Chemical Communications, vol. 46, no. 8, pp. 1317-1319, 2010.
[27] K. C. Popat, E. E. Swan, V. Mukhatyar, K. Chatvanichkul, G. K. Mor, C. A. Grimes and T. A. Desai, "Influence of nanoporous alumina membranes on long-term osteoblast response," Biomaterials, vol. 26, no. 22, pp. 4516-4522, 2005.
[28] R. E. Burrell, A. G. Naylor and A. M. Rosenfeld, "Thin Film Diagnostic". US Patent 5,124,172, 23 June 1992.
[29] G. Wypych, Handbook of Fillers 3rd Edition, ChemTec Publishing, 2010.
[30] J. Benesch, A. Askendal and P. Tengvall, "The determination of thickness and surface mass density of mesothick immunoprecipitate layers by null ellipsometry and protein 125iodine labeling," Journal of Colloid and Interface Science, vol. 249, no. 1, pp. 84-90, 2002.
[31] J. Vörös, "The Density and Refractive Index of Adsorbing Protein Layers," Biophysics Journal, vol. 87, no. 1, pp. 553-561, 2004.
[32] P. G. Sheasby and R. Pinner, The Surface Treatment and Finishing of Aluminum and its Alloys - 6th Edition, Finishing Publications Ltd., 2001.
[33] L. Chun-hung, "Why do rainbow-like colors appear on the surface of oil?," Physics World, [Online].
[34] "A single, integrated point-of-care testing solution," Abbott Point of Care Inc., 2017. [Online]






































