Pretreatment for powder and liquid coating is often described as a consecutive series of stainless-steel tanks, spray stages, and chemical process stations.
 Mario QuicenoWhen viewed schematically, it appears to be a linear system in which parts are cleaned, rinsed, converted, and dried.
Mario QuicenoWhen viewed schematically, it appears to be a linear system in which parts are cleaned, rinsed, converted, and dried.
However, once the process is running in production, it becomes a dynamic system in which chemistry, water quality, temperature, and dwell time interact continuously. For this reason, it is essential to maintain methodical control of all these variables. When they are properly balanced, the coating process can perform like clockwork.
The risk arises when these variables are not controlled. Failures may not appear immediately; they may manifest weeks or even months later. At that point, an investigation cycle begins, and the coating material or curing process is often blamed, even though the root cause lies earlier in pretreatment.
Understanding how chemistry, water quality, temperature, and dwell time operate—and how they interact—is important not only for engineers and quality personnel but also for coating applicators and operators. Quality checklists typically provide target ranges, yet they rarely explain what these values represent, why they matter, or how they influence one another.
This article presents these interactions practically and explains why they are critical to production performance.
Pretreatment as a System, Not a Checklist
One of the most common mistakes in pretreatment is managing parameters independently. Chemistry is adjusted and controlled via titration; controllers and sensors regulate temperature; and dwell time is defined by line speed. In several production lines I have observed, chemistry was tightly controlled, while water quality and line speed variability were overlooked, resulting in inconsistent coating performance despite “in-spec” titration results.
In real production conditions, however, increasing chemical concentration may require longer drying time. At the same time, higher drying temperatures can accelerate reactions and increase evaporation, unintentionally removing chemical components needed to properly seal the surface.
Similarly, poor water quality can neutralize chemistry that was previously adjusted and controlled. Effective pretreatment performance depends on the interaction of all variables under real operating conditions, not on isolated setpoints.
Chemistry: The Foundation of the Process
Pretreatment chemistry is designed to perform specific functions: modifying the metal surface, removing contaminants, and creating a layer that promotes coating adhesion and corrosion resistance.
Whether alkaline cleaners, acidic cleaners, phosphates, zirconium-based treatments, zero-water systems, or other emerging technologies are used, chemical activity at the substrate surface must be carefully controlled to complete the following functions before coating application:
- Cleaning
- Surface modification
- Adhesion promotion
- Corrosion resistance
In summary, the variables commonly assumed to be under control include chemical concentration, temperature, contact time, contamination loading, and water chemistry. As shown later, each of these variables plays a critical role.
In systems that use water for rinsing or as a carrier for chemical stages, water quality must meet certain requirements to avoid compromising the final product. Pretreatment water is often assumed to be neutral, whereas chemistry is considered the active component. In reality, water quality is just as important and must be monitored and controlled.
Key water quality parameters include:
- Hardness (calcium and magnesium)
- Total Dissolved Solids (TDS)
- pH and alkalinity
- Chlorides and sulfates
- Silica and iron
- Microbiological activity (in some systems)
Each of these factors significantly affects cleaning efficiency, rinsing effectiveness, and conversion-coating formation.
Hard Water and Chemical Efficiency
Hard water reduces cleaning efficiency by reacting with surfactants and additives, forming insoluble compounds that reduce cleaning power. In practice, operators often increase the concentration to compensate for hard-water effects, which can temporarily improve cleaning but creates long-term stability and maintenance issues.
In conversion coatings, hardness ions can compete with metal ions at the surface, affecting coating weight, uniformity, and crystal structure.
Rinse water is not merely a dilution medium. It directly affects:
- Residue carryover into subsequent stages
- Surface pH before conversion
- Uniformity of the conversion layer
- Long-term corrosion resistance
Temperature: Reaction Speed and Process Stability
Temperature influences pretreatment chemistry by affecting reaction kinetics and process stability. Most cleaning and conversion reactions accelerate as temperature increases, generally resulting in:
- Improved soil removal
- Faster conversion coating formation
- Reduced dwell time requirements
For this reason, many pretreatment systems specify minimum operating temperatures. However, higher temperatures are not always better. Excessive temperature can:
- Increase evaporation and unintentionally concentrate chemistry
- Increase drag-out losses
- Accelerate chemical degradation
- Increase energy consumption
- Reduce operator safety margins
I have seen lines where the temperature was raised to maintain throughput, only to find that chemical consumption and bath instability increased significantly within weeks. Temperature strongly interacts with dwell time. A process designed for long dwell time at moderate temperature can behave very differently when line speed increases and temperature is raised to compensate.
Dwell time is often defined as the number of seconds a part spends in a stage, but effective dwell time depends on how the part contacts the solution.
Spray vs. Immersion
In spray systems, dwell time is influenced by:
- Nozzle type and pressure
- Spray angle and coverage
- Part geometry and orientation
- Line speed consistency
In immersion systems, dwell time includes:
- Agitation
- Load density
- Trapped air
- Boundary layer effects
Simply slowing the line does not guarantee better pretreatment if coverage or agitation is inadequate.
There is also a point of diminishing returns. Once soils are removed or a conversion layer forms, additional dwell time provides little benefit and may cause overetching or non-uniform coatings.
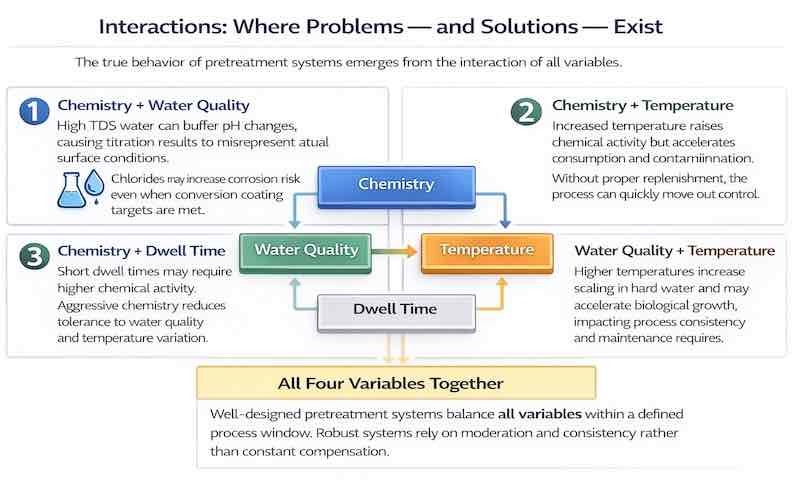
Interactions: Where Problems — and Solutions — Exist
The true behavior of pretreatment systems emerges from the interaction of all variables.
Chemistry + Water Quality: High TDS water can buffer pH changes, causing titration results to misrepresent actual surface conditions. Chlorides can increase corrosion risk even when conversion coating targets are met.
Chemistry + Temperature: Higher temperatures increase chemical activity but also accelerate reaction rates and increase contamination. Without proper replenishment, the process can quickly become uncontrolled.
Chemistry + Dwell Time: Short dwell times may require more aggressive chemistry, but aggressive chemistry reduces tolerance to water quality or temperature variation.
Water Quality + Temperature: Higher temperatures increase scaling in hard water and may accelerate biological growth in some systems, impacting consistency and maintenance requirements.
Well-designed pretreatment systems balance all four variables, so no single parameter is pushed to an extreme. Robust systems rely on moderation and consistency rather than constant compensation.
Why Failures Appear Downstream
Pretreatment-related failures rarely appear immediately. Adhesion loss, blistering, filiform corrosion, or underfilm corrosion often do not appear until after curing, assembly, shipping, or field exposure.
This delayed feedback leads teams to focus on:
- Powder formulation
- Cure schedules
- Application parameters
In root-cause investigations, pretreatment conditions are often reviewed last, even though they are often the root cause of downstream adhesion or corrosion failures. While important, many of these failures originate weeks earlier due to subtle imbalances among chemistry, water quality, temperature, and dwell time.
Practical Lessons
Stable pretreatment lines consistently follow these principles:
- Control variability before increasing aggressiveness: Stabilizing water quality and dwell time is often more effective than increasing chemical concentration.
- Measure what matters: Titration alone is insufficient. Monitor conductivity, temperature trends, rinse quality, and line speed consistency.
- Teach operators the “why”: When operators understand interactions, they make better decisions during disruptions.
- Tolerance design: Robust systems tolerate small deviations without immediate failure.

Zero-Water Pretreatment: Brief Overview
Zero-water pretreatment systems use a controlled-acidic resin and operate autonomously. Contaminants introduced with parts are continuously removed through filtration, while the resin absorbs process oils. The bath does not require dumping or recharging; only periodic replenishment is required.
Because no water or rinse stages are used, no wastewater is generated, no discharge permits are required, and the removed solids are not sent to landfill. Electrical consumption is minimal; spray pressures are typically 3-5 PSI, and the process uses solvents rather than water.
These solvents efficiently absorb oils and have extremely low vapor pressure. Airflow is controlled to prevent vapor release, and no VOCs, hazardous air pollutants, carcinogens, halogenated compounds, CFCs, or ozone-depleting substances are emitted.
Reported field results and supplier data indicate that zero-water pretreatment systems may achieve corrosion resistance exceeding iron phosphate on steel and over 2,000 hours with TGIC polyester topcoats on aluminum, with potential cost advantages from reduced footprint, gas use, wastewater treatment, and labor and energy demands.
Conclusion
Pretreatment is not chemistry in a laboratory beaker; it is chemistry in motion, under production pressure, influenced by water, heat, time, and human decisions. Successful finishing operations recognize that no parameter exists in isolation.
When chemistry, water quality, temperature, and dwell time are treated as an integrated system, pretreatment becomes predictable, repeatable, and resilient. When treated as independent checkboxes, the process becomes fragile, expensive, and reactive.
Understanding these interactions does not require advanced degrees in chemistry. It requires curiosity, observation, and respect for process complexity. Ultimately, a coating can only perform as well as the surface beneath it — and that surface is shaped long before powder or paint is applied. In my experience, the most reliable coating operations are those where operators and engineers understand not only the setpoints but also how each variable interacts with the others on the shop floor.
Water Quality Parameters and Recommended Ranges
| Parameter | Recommended Range |
| Total Hardness | < 50 ppm (ideal < 25) |
| TDS | < 300 ppm |
| pH | 6.5 – 8.5 |
| Alkalinity | < 100 ppm |
| Chlorides | < 50 ppm |
| Sulfates | < 100 ppm |
| Silica | < 10 ppm |
| Iron | < 0.3 ppm |
| Microbiology | < 10^4 CFU/mL |
Important note: These are general data from industrial coatings associations. Always consult your chemical supplier's specifications, but these ranges explain why problems arise when water is not properly calibrated or controlled.
Water control is not a chemical requirement; it is a quality requirement. Stable pretreatment begins with stable water.
Mario Quiceno serves as an Assembly Lead Hand at K-Hart Industries in Winnipeg, Canada. Before taking on this role, he had several years of experience in the powder-coating industry. He is an active member of the Chemical Coaters Association. Mario holds a degree in Mechanical and Manufacturing Engineering from Universidad Autónoma de Manizales, a Project Management certification, and an MBA from Universidad del Valle.
References
- Chemical Coaters Association International (CCAI). Pretreatment: A Foundation for Quality Finishing. CCAI Technical Series, Estados Unidos.
- Powder Coating Institute (PCI). Powder Coating: The Complete Finisher’s Handbook.
- Tercera edición, PCI, Estados Unidos.
- Golliver, J. The Powder Coach’s Playbook: Mastering the Art of Powder Coating.
- Powder-X Coating Systems, 2024.
- Spyrou, E. Powder Coatings: Chemistry and Technology.
- European Coatings Tech Files, tercera edición, 2012.
- Utech, B. A Guide to High-Performance Powder Coating. SME & AFP, 2002.
- Henkel Surface Technologies. Water Quality and Pretreatment Process Control.
- Publicaciones técnicas y documentos de aplicación industrial.
- WestChem Technologies Inc.: Pretreatments and advanced pretreatment solutions.
- WestChem Technologies technical information on metal finishing and pretreatment chemistries.































