In this theoretical treatment, the approach to the anodic aluminum oxide structure is based upon the premise that it is a self-assembled, highly ordered, nano-scale network of individual cells.
The individual cells are regarded from the point of origin to understand 1) how the oxide is ordered and 2) how the oxide network continues to grow after the substrate surface is reconstructed by oxide. By considering the conditions that surround the anodizing process, specifically, the effects of polarization and the applied current bias, a mechanistic approach to the formation and growth of the anodic aluminum oxide is presented.
1. Introduction
 Dr. Jude M. RungeOften the best way to understand the origin of something is to consider, in depth, its manufacturing process. What is involved in making anodic aluminum oxide (AAO)? Oxide growth requires diffusion of oxygen ions from the environment external to the aluminum substrate (the electrolyte) and counter diffusion of metal ions from the aluminum to the interface with the electrolyte through the forming oxide layer.
Dr. Jude M. RungeOften the best way to understand the origin of something is to consider, in depth, its manufacturing process. What is involved in making anodic aluminum oxide (AAO)? Oxide growth requires diffusion of oxygen ions from the environment external to the aluminum substrate (the electrolyte) and counter diffusion of metal ions from the aluminum to the interface with the electrolyte through the forming oxide layer.
In nature, when aluminum is exposed to the atmosphere, a passive oxide layer forms on the surface that is only a few nanometers thick. Oxidation proceeds on the exposed aluminum while the aluminum reacts, as the anode, with the atmosphere, which is functioning as the electrolyte. Without an applied electrical bias, the oxide layer grows in excess of a monolayer to an equilibrium thickness of a passive oxide layer, and stops with dissolution at the interface with the atmosphere and continued growth from the metal interface. The net reaction rate for passive layer formation is zero; aluminum is oxidized at the same rate as the oxide forms, identifying the formation of the passive layer as an equilibrium process.
In industry, the electrical bias is a necessary component of the manufacturing process to form a porous oxide, the applied current density, making it the unique component to manufacturing AAO, and an integral engineering component of the anodizing process. With applied constant current density, a steady state process, rather than equilibrium process develops, in which an infinite amount of aluminum ions are produced by way of oxidation while an infinite amount of hydroxide ions are produced by way of electrolysis of the electrolyte. Both electrons and ions carry the charge for the oxidation reaction through the anode, which polarizes the surface. Polarization develops an ordered charge density on the anode surface that overrides the energy of surface phenomena, which typically determine the points at which the oxide nucleates. Polarization not only sets nucleation, it produces regular spacing of the oxide nuclei, which develop into discrete oxide elements that comprise the highly ordered AAO. Therefore, surface polarization is not only key to the organization of charge on the surface; it is the source for the structural order of the AAO.
As the AAO grows and develops, it becomes a functional part of its own manufacturing process. With continued applied current density, polarization effects continue to impact how the AAO structure develops. The interfaces that bracket the oxide become critical boundaries to consider as they produce unique impact on continuous oxide growth. The aluminum anode is consumed by the oxide, and as the surface is reconstructed by oxide, growth stress develops at the substrate-oxide interface that is countered by polarization forces at the oxide-electrolyte interface. Upon impingement of the ordered oxide elements, the unique hexagonal structure of the anodic oxide results, and with continued oxide growth, electrostriction forces enable the oxide to grow in the direction of the applied current density while polarization forces stabilize and maintain the central pore that comprises the columnar cell that develops from each oxide element comprising the AAO.
This paper provides basic insight to the growth and development of anodic aluminum oxide as a self-assembled, highly ordered, nano-scale network of individual electrochemical cells. By regarding the cells from their point of origin, the following details of oxide growth are explained: how the oxide is ordered, how the network continues to grow after the surface is reconstructed by oxide, and how the unique structural characteristics of the AAO develop and function. The role of substrate polarization and the applied current density are presented as the basis for this unique mechanism. While the reality of anodizing industrial alloys mandates accounting for the effects of alloying elements and substrate defects on the anodizing process, this paper focus only on the mechanism for nucleation and growth of anodic oxide from an ideal aluminum surface.
2. Anodic Oxide Nucleation and Growth
2.1. Surface Polarization and Oxide Nucleation
By definition, the anodizing process requires the surface has a net positive charge for oxidation to proceed. As a metallic substrate for anodizing, aluminum has “free charges” that are able to move around; some electrons are not attached to any particular atom, and some comprise tightly bound electron clouds that function as interstitial bonding arms on the FCC structure. In this respect, a metal can behave like a conductor and a semiconductor. In aluminum, the bonding arms are comparatively long, which enables easy alloying and ductility. When aluminum is placed in an electric field, the electron cloud that comprises each bonding arm on the FCC lattice is distorted instantaneously so that each behaves like a charged dipole, orienting the dipoles so that the bulk of the substrate functions to maintain a neutral charge, while the surface exhibits a net positive or net negative charge. In anodizing, the distortion and orientation of the charges in the aluminum, which produces charge neutrality in the substrate bulk, simultaneously polarizes the surface, producing an ordered charge density comprised of many discrete, evenly spaced positive charges over the surface. 1,2 See Figure 1.
![Figure 1: When the substrate is placed in an electric field, E, the charges on the FCC atomic lattice become oriented such that the bulk of the substrate maintains charge neutrality; however, when anodically polarized, the surface has a net positive charge. [2]](/images/images/runge/polarization/1.jpg) Figure 1: When the substrate is placed in an electric field, E, the charges on the FCC atomic lattice become oriented such that the bulk of the substrate maintains charge neutrality; however, when anodically polarized, the surface has a net positive charge. [2]
Figure 1: When the substrate is placed in an electric field, E, the charges on the FCC atomic lattice become oriented such that the bulk of the substrate maintains charge neutrality; however, when anodically polarized, the surface has a net positive charge. [2]
With the applied current density, electrons initially carry all of the electric current in the aluminum substrate. If we consider first the situation of an anodic oxide growing on an ideal aluminum substrate: all cation interstitials on the anode lattice will be of the same size, and carry the same charge, because they are all aluminum. The ratio of electrons to atomic (ionic) currents is high. Electrotransport, which in the case of an ideal aluminum substrate, is comprised only of aluminum ions, results from the impact of the flux of electrons on interstitial atoms on the fcc lattice. These interstitial atoms make large diffusive jumps. The moving electrons hit the interstitial atoms, and, transferring their momentum, bias the diffusive jumps in the direction of the applied current density. The continued biased movement of electrons movement also inhibits jumps from occurring in the opposite direction; consequently the substrate surface is flooded with aluminum ions and is positively charged as an anode.3
Polarization, as a response to the applied current density, sets the charge density at the surface during anodizing. As free aluminum ions in the metal are being driven to the interface, simultaneously, electrons are ejected from the surface into the electrolyte, causing electrolysis. Polarization also drives the formation of evenly spaced Al3+ nodes across the anode surface; the spacing of the nodes reflects the charge density and they are therefore symmetric with respect to one another. During electrolysis, most of the electrons are consumed by hydrogen ions in the electrolyte, and travel to the cathode where they evolve as hydrogen gas. The rest of the electrons are pulled back toward the positive charge distribution at the surface. This is because not all of the electrons have enough energy to immediately escape the charge density at the surface, due to the presence of so many metal ions (primarily Al3+), which draws them back toward the surface. Electrons therefore end up oscillating over the positive charge distribution, producing a charge density wave at the substrate-oxide interface, complementary to the Al3+ nodes on the surface of the substrate. See figure 2.
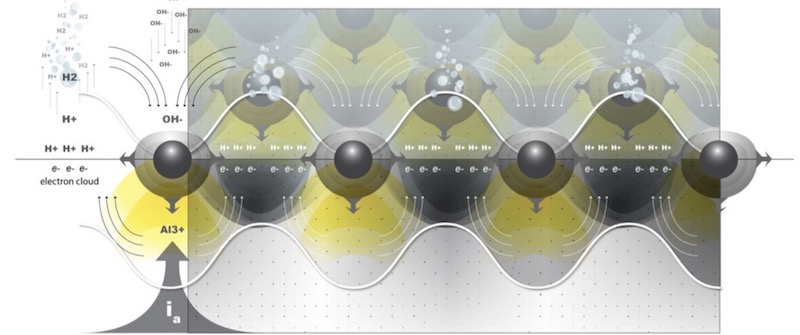 Figure 2: Schematic of a current density surface wave, which is the result of surface polarization. The current density wave at the substrate surface is the result of electrons, ejected from the surface, interacting with the positive surface charge due to anodic polarization.
Figure 2: Schematic of a current density surface wave, which is the result of surface polarization. The current density wave at the substrate surface is the result of electrons, ejected from the surface, interacting with the positive surface charge due to anodic polarization.
The resultant condition is called anodic polarization, which represents the driving force for aluminum corrosion and in the specific case of anodizing, for oxidation. The oxidation reaction occurs at the substrate surface as the applied current density pushes aluminum ions and electrons from the surface, occurring primarily where the reaction is organized by the resultant charge density wave, set by polarization of the surface. The net polarization is in excess of the equilibrium oxidation potential for aluminum, and its magnitude governs the spacing of the charge at the surface, which is on the order of several nanometers.
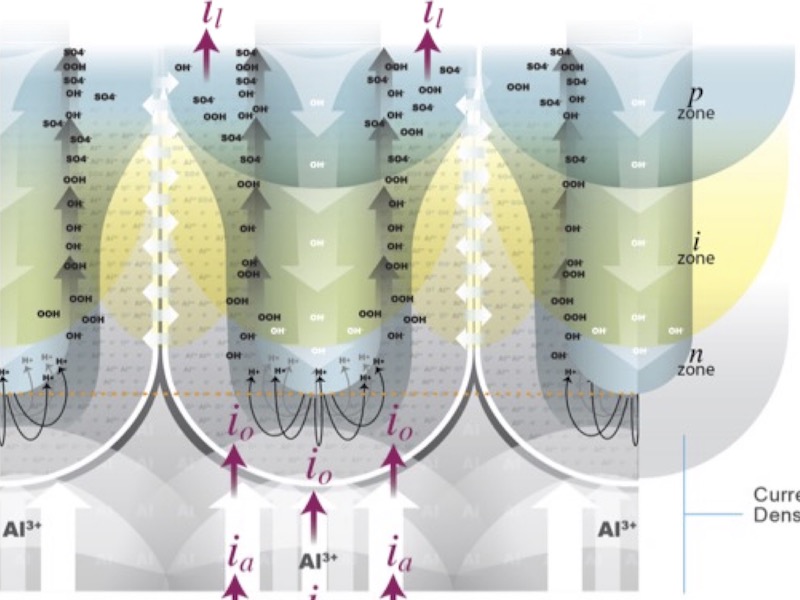
The charge density wave at the substrate surface is maintained by continued polarization as the applied current density drives the oxidation reaction: the continuous movement of aluminum ions, as well as other metallic ions (in the case of an alloy substrate) and the ejection of electrons from the surface. The electrons ejected from the surface also interact with each other, maintaining a free electron cloud above of the positive charge distribution at the polarized surface. Electrons complete the next step in the anodizing circuit as they react to decompose (electrolyze) the electrolyte: electrons are consumed by the reduction of water to form hydrogen ions, which, because they are positively charged, proceed to the cathodes to complete the anodizing circuit, forming hydrogen gas. Negatively charged hydroxide ions remain and are trapped at the interface with the anode.
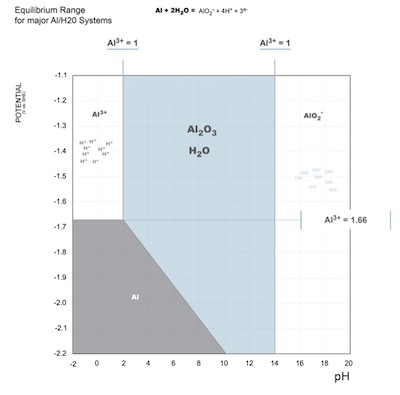 Figure 3: Modified Pourbaix diagram, stability boundaries are placed to reflect the oxidation potential as -1.66V for all pH. Under non-equilibrium conditions, an infinite amount of Al3+ions is available to form Al2O3, which increases the stability range of the oxide. Nevertheless, anodizing electrolytes have a pH <<2, yet anodic oxides are manufactured industrially every day. Polarization, set by the applied current density of the anodizing process, provides the conditions that locally increase the pH, enabling stable oxide to form. Because the electric field effects are concentrated at polarized centers across the surface, the electron cloud tapers off at interstitial spaces between charge density concentrations, and hydroxide ions become concentrated at saddles in the charge density wave the interface with the anode. The formation of periodic areas of hydroxide ion concentration produces a locally higher pH, relative to the pH of the electrolyte, in the saddles of the charge density wave adjacent to the polarized nodes, which are rich in Al3+ ions. It is here, with the simultaneous production of hydrogen gas and hydroxide ions in the electrolyte at the interface with the substrate, that the formation of anodic aluminum oxide nuclei proceeds at discrete, evenly spaced points on the substrate surface where Al3+ ions have accumulated due to polarization. This reaction governs the oxygen pressure at the interface with the substrate by the reduction of water at the surface. Most important, the charge density wave produces the variation in pH local to the surface, necessary to produce the anodizing conditions that enable stable oxide formation under conditions that are not in line with the stability boundaries for aluminum oxide set forth by the Pourbaix Diagram.4 See Figure 3.
Figure 3: Modified Pourbaix diagram, stability boundaries are placed to reflect the oxidation potential as -1.66V for all pH. Under non-equilibrium conditions, an infinite amount of Al3+ions is available to form Al2O3, which increases the stability range of the oxide. Nevertheless, anodizing electrolytes have a pH <<2, yet anodic oxides are manufactured industrially every day. Polarization, set by the applied current density of the anodizing process, provides the conditions that locally increase the pH, enabling stable oxide to form. Because the electric field effects are concentrated at polarized centers across the surface, the electron cloud tapers off at interstitial spaces between charge density concentrations, and hydroxide ions become concentrated at saddles in the charge density wave the interface with the anode. The formation of periodic areas of hydroxide ion concentration produces a locally higher pH, relative to the pH of the electrolyte, in the saddles of the charge density wave adjacent to the polarized nodes, which are rich in Al3+ ions. It is here, with the simultaneous production of hydrogen gas and hydroxide ions in the electrolyte at the interface with the substrate, that the formation of anodic aluminum oxide nuclei proceeds at discrete, evenly spaced points on the substrate surface where Al3+ ions have accumulated due to polarization. This reaction governs the oxygen pressure at the interface with the substrate by the reduction of water at the surface. Most important, the charge density wave produces the variation in pH local to the surface, necessary to produce the anodizing conditions that enable stable oxide formation under conditions that are not in line with the stability boundaries for aluminum oxide set forth by the Pourbaix Diagram.4 See Figure 3.
Figure 3: Modified Pourbaix diagram, stability boundaries are placed to reflect the oxidation potential as -1.66V for all pH. Under non-equilibrium conditions, an infinite amount of Al3+ions is available to form Al2O3, which increases the stability range of the oxide. Nevertheless, anodizing electrolytes have a pH <<2, yet anodic oxides are manufactured industrially every day. Polarization, set by the applied current density of the anodizing process, provides the conditions that locally increase the pH, enabling stable oxide to form.
The spacing and stability of the centers of concentrated oxide nucleation are governed by two related mechanisms: 1) spacing is governed by the surface polarization (voltage) that is the result of the charge density at the surface applied by the applied current density, set by the external power supply; and 2) stability is assured by way of the periodic accumulation of aluminum ions over the surface that are in alignment with concentrations of OH- ions in the charge density wave, producing energetically favorable reaction sites for the formation of stable oxide nuclei, rather than at other surface defects that might be preferred if the substrate wasn’t polarized. Evenly spaced agglomerates of oxide molecules are formed at the sites of aluminum ion accumulation, which immediately develop into evenly spaced oxide nuclei. See Figure 4.
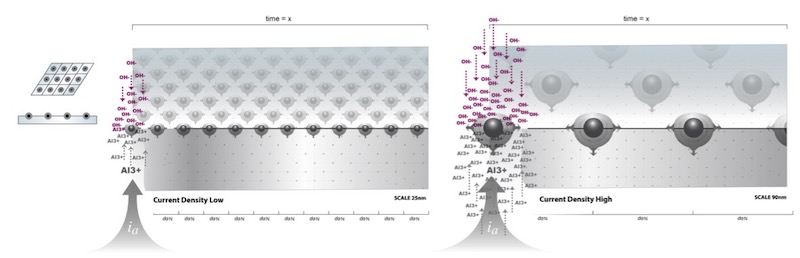 Figure 4: Spacing of oxide nuclei is governed by the surface polarization (voltage) that is the result of the charge density at the surface applied by the applied current density, set by the external power supply. At low applied current density, many equally spaced nuclei form due to the reduced potential response polarizing the surface. At high current density, the converse is true.
Figure 4: Spacing of oxide nuclei is governed by the surface polarization (voltage) that is the result of the charge density at the surface applied by the applied current density, set by the external power supply. At low applied current density, many equally spaced nuclei form due to the reduced potential response polarizing the surface. At high current density, the converse is true.
2.2. Surface Reconstruction
If one considers the surface polarization in terms of how the charge density is organized across the surface, it is easy to visualize how the concentration of aluminum ions accumulates at polarized nodes, and then tapers interstitially, between symmetric nodes. The charge density wave corresponds to and complements polarization, and as electrolysis continues, hydroxide ions are concentrated with aluminum ions at each polarized node, and move with the oxide growth front as it proceeds into the substrate. This situation provides the concentration gradient for continuous lateral growth and consumption of the aluminum by the developing oxide layer. Growth occurs outward from each polarized oxidation center; reconstructing the surface by forming progressively larger circular oxide flakes that come together to form a hexagonal network. In his seminal work, performing stop-action-microscopy of anodic oxide growth, Csokan documented lateral growth, describing it as occurring in continuous waves of secondary oxide growth.5 See Figure 5.
With total surface reconstruction, the oxide network has consumed the entire anode surface and each flake comprising the network constrains its nearest neighbor, producing a hexagonal surface structure. However, because the oxidation reaction is initiated at points of symmetry set by polarization, more aluminum is naturally consumed at these centers due to the increased amount of aluminum ions, to a depth proportional to the applied current density, yielding the hemispherical base for each oxide element. Therefore, radius of each oxide element is not only the radius and side of each hexagon comprising the surface, but is also the radius of the hemisphere at the base of each oxide element, which remains constant, representing the transient thickness of the oxide layer at the point of total surface reconstruction.
2.3. Electrostriction and Oxide Growth
In an electrochemical reaction driven by an external power supply, instability of the oxide increases rather than stops with total surface reconstruction.6 The impingement of the polarized, dynamically growing oxide flakes introduces an additional, non-equilibrium component to the stress in the growing oxide by way of electrostriction, creating electrostriction stress. Continued application of the current density produces continued oxide growth, causing neighboring oxide flakes to actually push against one another, continually producing more mechanical strain in the individual oxide flakes. The uniformity of the compressive stress across the surface prevents the individual oxide flakes that comprise the growing network from sliding over one another or collapsing on top of one another.
![Figure 5: Progressive oxide development over a period of ~10 seconds in a 5% sulfuric acid electrolyte with 36 – 38V at ± 1oC. In dilute electrolytes at low current density, nucleation is preferential rather than precisely ordered by polarization. In the first photomicrograph (upper left-hand corner), preferential nuclei, corresponding to the rolling direction of the substrate, are observed in the first half-second of exposure after switching on the current, and grow outward, becoming circular oxide “flakes” which ultimately reconstruct the anode surface. [5]](/images/images/runge/polarization/5.jpg) Figure 5: Progressive oxide development over a period of ~10 seconds in a 5% sulfuric acid electrolyte with 36 – 38V at ± 1oC. In dilute electrolytes at low current density, nucleation is preferential rather than precisely ordered by polarization. In the first photomicrograph (upper left-hand corner), preferential nuclei, corresponding to the rolling direction of the substrate, are observed in the first half-second of exposure after switching on the current, and grow outward, becoming circular oxide “flakes” which ultimately reconstruct the anode surface. [5]
Figure 5: Progressive oxide development over a period of ~10 seconds in a 5% sulfuric acid electrolyte with 36 – 38V at ± 1oC. In dilute electrolytes at low current density, nucleation is preferential rather than precisely ordered by polarization. In the first photomicrograph (upper left-hand corner), preferential nuclei, corresponding to the rolling direction of the substrate, are observed in the first half-second of exposure after switching on the current, and grow outward, becoming circular oxide “flakes” which ultimately reconstruct the anode surface. [5]
In order to explain electrostriction and its effect on the total AAO structure, it is best to consider the development of stress in the oxide from the perspective of the single cell within the oxide network. This enables correlation to experimental data as well as explanations for how structural features of the anodic oxide, such as the central pore and knit-lines develop. Stress due to electrostriction is a direct response to the direction of the applied current density, producing change in the structure after the AAO has reconstructed the anode surface, as the oxide elements impinge. Prior to surface reconstruction, as long as the thermodynamic growth stress produced by the applied current density drives the oxidation reaction by way of the development of interfacial resistance, there can be no deformation or reorientation of the total AAO structure on isotropic aluminum. In fact, because there is no electrostriction stress in the oxide prior to surface reconstruction, the formation of the rounded bases of each oxide element is enabled as the AAO naturally takes on the shape of least thermodynamic stress. Constraint of the individual oxide elements, or flakes, produces the hexagonal pattern of the self-assembled cells that reconstruct the aluminum substrate surface, and is the source for stress in the oxide due to electrostriction. See Figure 6.
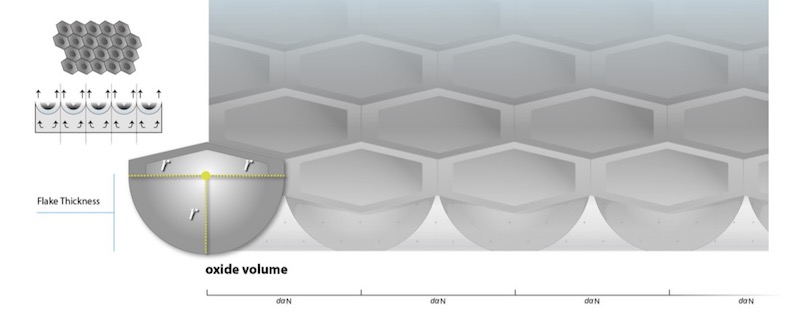 Figure 6: Constraint of the individual oxide elements, or flakes, produces the hexagonal pattern of the self-assembled cells that reconstruct the aluminum substrate surface. It is also the source for stress in the oxide due to electrostriction, which, as oxide growth continues, is counter-balanced by polarization forces, forming the central pore.
Figure 6: Constraint of the individual oxide elements, or flakes, produces the hexagonal pattern of the self-assembled cells that reconstruct the aluminum substrate surface. It is also the source for stress in the oxide due to electrostriction, which, as oxide growth continues, is counter-balanced by polarization forces, forming the central pore.
2.4. Development of the Anodic Oxide Structure
The tensile component of the electrostriction stress, related to the dielectric nature of the forming oxide, also referred to as dielectrostriction, develops through the thickness of the growing oxide, orthogonal to the substrate.7 The contribution of additional tensile stress to the total stress in the oxide induces uniform deformation in each oxide flake at the point of impingement with the nearest neighbor flakes, which proceeds as growth continues into the aluminum substrate. Electro-mechanical strain develops at each interface of each element comprising the growing oxide. Deformation occurs in each oxide element comprising the AAO network, and causes the edges of the flakes flow in the direction of the applied current, away from the substrate. As the oxide growth front proceeds into the aluminum substrate, the edges of each impinging flake become seams as “dimples” begin to form at the center of each flake in the developing oxide network.
Polarization forces, acting on the inside of dimple at the center of each individual flake, push against the mechanical forces in the oxide; as they repel one another from opposing oxide surfaces, a cup-like cell forms. As mechanical forces build in each oxide flake or element, electrostriction effects increase. The inside surface of each cup remains erect, sustained by the polarization voltage that established its initial center. Polarization forces act to keep each cup in compression with respect to one another. The surface of the oxide is also in compression with respect to the plane of the substrate. The alignment of the dipoles in the ionic solid and ionic conduction through the thickness of the oxide, due to the applied current density and the continued oxidation reaction, act to maintain dielectrostriction, and therefore the central pore in each columnar cell of the oxide network.
Prior to surface reconstruction, electrons and hydrogen ions are primarily expelled along the gaps between the oxide flakes. With surface reconstruction, the initial network of oxide flakes impinges on the surface of the oxide flake that becomes the outer diameter of each columnar cell. Electric field effects in the forming AAO structure are at a maximum from the polarized centers from which each cell of the oxide network originates, and taper to the edges of each oxide element (flake) where the cells impinge.8 With continued imposition of the current bias, each individual oxide flake constantly exerts lateral and downward forces against its nearest neighbor, forming distinct seams that circumscribe each columnar cell in the oxide network.
As the seams form, ion exchange occurs through the oxide structure in order to maintain the anodizing reaction. The dimensional uniformity of the oxide cells, primarily the cell wall thickness (including the base) and pore diameter depend upon continuity of the oxidation reaction. Electrostriction forces produce internal mechanical strain between the cells that stimulate mass transport across each seam, “knitting” the structure of the network together. Analysis shows the cell walls are fused together, implying that diffusion and/or mass transport occurs across the outer wall of each cell, “knitting” the structure together; hence this structural feature was given the name “knit-line”. Knit-lines are a product of AAO growth, and continue to form as long as the current is applied and the oxidation reaction proceeds.9 See figure 7.
![Figure 7: 2-D Schematic representing the “network in the network” of the anodic oxide, comprised of individual columnar cells, each with a central pore and circumscribed by a knit-line. Dynamic ion exchange occurs along the central pore. Knit-lines join each cell to its nearest neighbor. [10]](/images/images/runge/polarization/7.jpg) Figure 7: 2-D Schematic representing the “network in the network” of the anodic oxide, comprised of individual columnar cells, each with a central pore and circumscribed by a knit-line. Dynamic ion exchange occurs along the central pore. Knit-lines join each cell to its nearest neighbor. [10]
Figure 7: 2-D Schematic representing the “network in the network” of the anodic oxide, comprised of individual columnar cells, each with a central pore and circumscribed by a knit-line. Dynamic ion exchange occurs along the central pore. Knit-lines join each cell to its nearest neighbor. [10]
3. Conclusion
Polarization is the result of the applied current density set by the anodizing process; therefore, the size and number of polarized centers across a substrate surface are governed by its magnitude. Since polarization affects the surface at the atomic level, several uniformly spaced concentrations of oxide nuclei centers form per grain aluminum. For example, oxides formed at lower current density processes, such as decorative anodic oxides, have correspondingly lower surface potentials (reduced polarization forces), and exhibit more centers of oxide nuclei per unit grain. This is because with a lower applied current density there is less repulsion of accumulated like charges across the surface and fewer aluminum ions available to react at each node; therefore, more smaller nuclei form per crystallographic grain at the surface of the anode at lower current densities. Conversely, oxides formed at higher current densities (higher voltage), such as hard anodic oxides, exhibit fewer nuclei per unit grain aluminum because polarization forces set by the higher current density set them further apart. There are fewer, larger nuclei per unit grain at the anode surface with a higher applied current density, with more repulsion of accumulated like charges and more aluminum ions available to react. Summarizing: the spacing of oxide nuclei is closer together at lower charge (current) densities, applied current density, as in decorative or Type II anodizing (average pore center-to-pore center spacing = 25nm) and further apart at higher current densities, applied current density, as in hard or Type III anodizing (average pore center-to-pore center spacing = 80 – 100nm).10
The effects of electrostriction are permanent and uniform on the shape of each cell in the oxide network. Each cup-like cell grows to form the individual columnar cells that comprise the oxide network by way of continued oxide growth and deformation and oxide flow due to electrostriction forces counter by polarization effects. The polarization effects on the inside of the cup surface, reflecting the resultant corrosion potential (voltage), also stabilize the central pore and dictate the pore diameter. The thickness of the base and wall of each cell in the network is proportional to the voltage. Experimental data from constant current anodizing studies show that stress due to electrostriction increases linearly with the voltage response. The consistency and reliability with which anodic alumina can be manufactured shows that the relationship between electrostriction and polarization render a tunable structure of precise dimensions.
Acknowledgements: the author would like to acknowledge Ms. Joy Kaufman of JoyJoy Creations for the schematic illustrations.
 "The Metallurgy of Anodizing Aluminum" is available at https://link.springer.com/book/10.1007/978-3-319-72177-4Dr. Jude Mary (Judy) Runge’s career as a metallurgical engineer and surface finishing expert spans almost 40 years in industrial, government and academic professional settings. Beginning in 1982 at Northrop Corporation, Defense Systems Division, and culminating today as a Principal Engineer, Surface Finishing at Apple (since 2019), she is recognized internationally as a nonferrous specialist focussing on materials engineering problem solving that utilizes her expertise as a surface scientist and manufacturing process engineer, providing characterization for product development, failure analysis and metallurgical support to the aluminum finishing industry. She is well known for her work in anodizing that led to a new theoretical treatment for porous oxide formation. Dr. Runge received her Ph.D. in metallurgy at the University of Illinois at Chicago under Dr. Michael McNallan. A tireless educator, Dr. Runge has authored numerous papers and given seminars worldwide; she is the Education Chair for the Aluminum Anodizers Council since 2008. Her book, “The Metallurgy of Anodizing Aluminum”, published by Springer Nature in 2018, is one of her biggest personal achievements. Judy is third of nine children and the first in her family to attend college/university. She is the mother of 4 and grandmother of 8. She believes her success is the result of great personal grit and passion for science, which enabled her hard work and very often, hard decisions. She owes a great deal of her career to her mother, who continuously challenged and supported her. She is grateful to her husband, Thomas Nussbaum, for his love and support and for admitting always how proud he is of her.
"The Metallurgy of Anodizing Aluminum" is available at https://link.springer.com/book/10.1007/978-3-319-72177-4Dr. Jude Mary (Judy) Runge’s career as a metallurgical engineer and surface finishing expert spans almost 40 years in industrial, government and academic professional settings. Beginning in 1982 at Northrop Corporation, Defense Systems Division, and culminating today as a Principal Engineer, Surface Finishing at Apple (since 2019), she is recognized internationally as a nonferrous specialist focussing on materials engineering problem solving that utilizes her expertise as a surface scientist and manufacturing process engineer, providing characterization for product development, failure analysis and metallurgical support to the aluminum finishing industry. She is well known for her work in anodizing that led to a new theoretical treatment for porous oxide formation. Dr. Runge received her Ph.D. in metallurgy at the University of Illinois at Chicago under Dr. Michael McNallan. A tireless educator, Dr. Runge has authored numerous papers and given seminars worldwide; she is the Education Chair for the Aluminum Anodizers Council since 2008. Her book, “The Metallurgy of Anodizing Aluminum”, published by Springer Nature in 2018, is one of her biggest personal achievements. Judy is third of nine children and the first in her family to attend college/university. She is the mother of 4 and grandmother of 8. She believes her success is the result of great personal grit and passion for science, which enabled her hard work and very often, hard decisions. She owes a great deal of her career to her mother, who continuously challenged and supported her. She is grateful to her husband, Thomas Nussbaum, for his love and support and for admitting always how proud he is of her.
References
- Runge, J., The Metallurgy of Anodizing Aluminum, Springer Scientific Publishing, New York, 2017.
- Rana, F., “Polarization”, Lecture 7, ECE 303, Department of Electrical and Computer Engineering, Cornell University, 2007.
- Shewmon, P., Diffusion in Solids, TMS Publication, Salem, Massachusetts, 1989.
- Jones, Denny A., Principles and Prevention of Corrosion, 2nd Edition, Prentice Hall, Upper Saddle River, NJ, 1996.
- Csokan, P., “Nucleation Mechanism in Oxide Formation During Anodic Oxidation of Aluminum”, Advances in Corrosion Science and Technology, ed. M.G. Fontana, Plenum Press, New York, 1980.
- Barkey, D., McHugh, J., “Pattern Formation in Anodic Aluminum Oxide Growth by Flow Instability and Dynamic Re-Stabilization”, Journal of the Electrochemical Society, 2010, Vol. 157, issue 11, C388 – C391.
- Van Overmeere, Q., Blaffart, F., La Mantia, F., Di Quarto, F., Proost, J., “Electromechnaical coupling in anodic niobium oxide: Electric field-induced strain, internal stress, and dielectric response”, Journal of Applied Physics 111, 113529 (2012).
- Parkhutik, V.P., Shershulsky, V.I., “Theoretical modeling of porous oxide growth on aluminium”, J. Phys. D: Appl. Phys. 25 (1992)1258- 1263.
- Runge, J. M., Pomis, A. J., “Anodic Oxide Film Formation Relating Mechanism to Composition and Structure”, Proceedings ASST, Manchester, England, 2000.
- Runge, J., “Formation of Porous Anodic Oxide Finishes: A New Approach and Theory”, from the conference proceedings of Aluminium 2000, Florence, 2007.