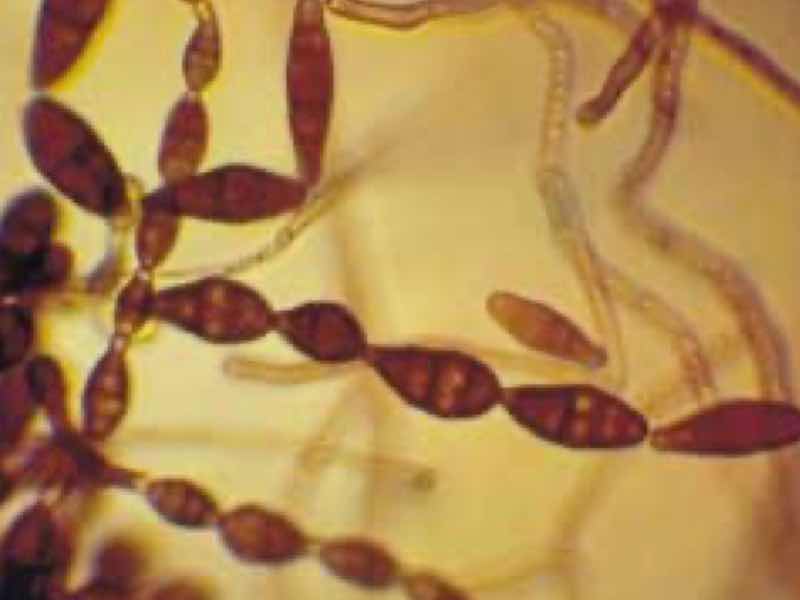
Biological contamination in tank-line processes can affect part quality, cycle time, auxiliary equipment corrosion, and worker safety.
 W. John FullenThe processes affected are varied and can occur in a wide range of pH conditions. At Boeing, bio-contaminant organisms, observed during processing, have been fungal (Alternaria, Fusarium, and Penicillium species) and bacterial (Pseudomonas species).
W. John FullenThe processes affected are varied and can occur in a wide range of pH conditions. At Boeing, bio-contaminant organisms, observed during processing, have been fungal (Alternaria, Fusarium, and Penicillium species) and bacterial (Pseudomonas species).
“The bio-films that form on the surface of virtually all structural metals and alloys immersed in aqueous environments have the capability to influence the corrosion of those metals and alloys. This influence derives from the ability of the organisms to change environmental variables, including oxidizing power, temperature, and concentration.” (Ref. 1.)
Because these organisms are thought to be air-borne and ubiquitous, a quite effective means of bio-contamination control can be to use covers for each process and rinse tank. Whereas, this is a good design characteristic for planning a new tank-line, it can be rather expensive and disruptive to do so after a line is in operation. At Boeing, there are several tank-lines that are either too large for covers or predate the conventional wisdom of incorporating that aspect in the design. Therefore, controlling the growth of what will inevitably enter these process and rinse solutions is the next most suitable alternative.
The following case studies exemplify situations where bio-contamination made it untenable to continue processing under existing conditions; they also show what remedies were sought, explain the required engineering testing, and demonstrate the implementation results.
Magnetic Particle Inspection
 Magnetic particle inspection.The Magnetic Particle Inspection (MPI) operation1 was hindered by a short bath life of five to seven days. An evaluation of samples of the bath solution confirmed findings of reduced indication (fluorescent magnetic particles) sensitivity and reduced settling volume (< 30%), which necessitate the frequent dumps.
Magnetic particle inspection.The Magnetic Particle Inspection (MPI) operation1 was hindered by a short bath life of five to seven days. An evaluation of samples of the bath solution confirmed findings of reduced indication (fluorescent magnetic particles) sensitivity and reduced settling volume (< 30%), which necessitate the frequent dumps.
Initially, inductively coupled plasma (ICP) was used to analyze suspect contamination (underneath the “tail-stop”) along with control specimens of the bath solution. This analytical method identified the presence of metals. The results revealed higher levels of Fe (iron), which is consistent with an evaporated bath solution containing Fe2O3 (iron oxide). The bath was contaminated by soils, such as high temperature grease, that are not easily removed by aqueous cleaning methods. However, ICP did not detect the presence of Mo (molybdenum), which is typically in the form of molybdenum disulfide in high temperature greases.
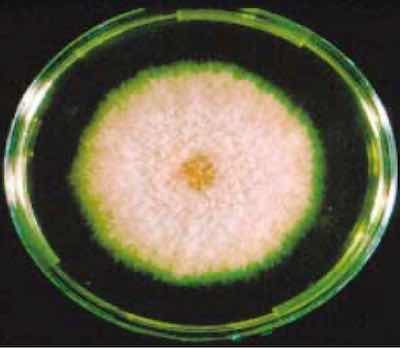 Fusarium fungus culture.Associated rinse tanks were found to be not well agitated and appeared to have low flow rates. This could cause the removed soils to be carried over on parts cleaned just prior to MPI. QA records show that the rinse tanks have a relatively low amount of total dissolved solids (TDS). Additionally, rinse water was added to fresh MPI bath solution samples with no characteristic adverse visual effects (cloudy solution). Therefore, this potential cause was eliminated.
Fusarium fungus culture.Associated rinse tanks were found to be not well agitated and appeared to have low flow rates. This could cause the removed soils to be carried over on parts cleaned just prior to MPI. QA records show that the rinse tanks have a relatively low amount of total dissolved solids (TDS). Additionally, rinse water was added to fresh MPI bath solution samples with no characteristic adverse visual effects (cloudy solution). Therefore, this potential cause was eliminated.
Furthermore, conditions of bath conditions exhibited a lack of “water break free” on the inspected parts and flocculation of the Fe2O3 particles to the sidewalls of the tank. These observations indicate that the “conditioner” (non-ionic surfactant) did not perform, as it should. Adding more WA2B did prolong the tank life but soon thereafter a dump was required.
Gas chromatography/mass spectrometry (GC/MS) was performed on old and new MPI bath solution samples. This analytic method identified a presence and quantity of organics. From results of GC/MS analysis and the physical observation of bath conditions and processed parts, we determined that the conditioner was being depleted. Subsequently, a bacterial microorganism (Pseudomonas) was confirmed to be present in the MPI bath that could be responsible for consuming this non-ionic surfactant. These bacteria are clinically important because they are resistant to most antibiotics and are capable of surviving in conditions that few other organisms can tolerate. They also produce a slime layer that is resistant to protection by white blood cell attack (phagocytosis). P. aeruginosa (Pseudomonas) prefers to inhabit moist environments but it can survive in a medium that is as deficient as distilled water.2
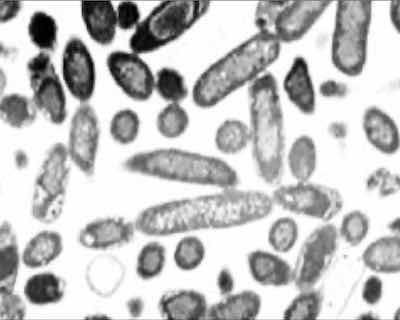 Pseudomonas bacteria.Initial corrective action efforts involved increasing the MPI solution pH to the highest level allowed per BSS 70404. This pH modification was attempted based on the recommendation of an outside subject industry expert, theorizing that the bacteria would not be prolific at a higher pH. However, no improvement in bath life was observed by increasing the pH to 10.0—the maximum allowed. Consequently, improvement activities concentrated on identifying a new biocide.
Pseudomonas bacteria.Initial corrective action efforts involved increasing the MPI solution pH to the highest level allowed per BSS 70404. This pH modification was attempted based on the recommendation of an outside subject industry expert, theorizing that the bacteria would not be prolific at a higher pH. However, no improvement in bath life was observed by increasing the pH to 10.0—the maximum allowed. Consequently, improvement activities concentrated on identifying a new biocide.
The first biocide (type) tried was initially successful but operators immediately noticed a disagreeable odor. After a relatively short amount of time this biocide was rendered ineffective and its use was discontinued.
In collaboration with the Boeing Microbiology lab and the MPI solution supplier,5 a quaternary ammonium biocide6 was identified. The initial dose of this biocide was ~30 mL (1.0 fl oz.) to achieve a concentration of ~400 ppm. Weekly maintenance adds of ~15 mL (0.5 fl oz) were performed thereafter. The Boeing Microbiology lab analyzed samples before the initial dose and ~36 hours later. Biological monitoring continued for ~4 weeks to help establish a reduced add frequency. This testing established that the biocide was working well. The reduction of biological contamination went from 2.8x106 cfu/mL to 0 cfu/mL. Consequently, the tank dump frequency was extended ~tenfold and the add frequency was reduced to biweekly. Finally, operators were informed that if the settling test (per BSS 7040) appeared to be marginal, adds of the conditioner could be made to further extend the life of this tank.
The resulting annual cost savings was estimated to be about $13,000 for this single small operation.
Alkaline Etch Rinse
 60,000-gal anodize tank.The second rinse of a double counter-current (DCC) rinse following the triethanolamine (TEA) alkaline etch process7 is often hampered by bio-contamination. A biomass grows on the tank walls and then breaks off and floats free in the tank. This biomass can adhere to the aluminum parts in process and this disrupts the operator by requiring a manual wash or, if un-noticed, can interfere with the acid desmutting step that follows.
60,000-gal anodize tank.The second rinse of a double counter-current (DCC) rinse following the triethanolamine (TEA) alkaline etch process7 is often hampered by bio-contamination. A biomass grows on the tank walls and then breaks off and floats free in the tank. This biomass can adhere to the aluminum parts in process and this disrupts the operator by requiring a manual wash or, if un-noticed, can interfere with the acid desmutting step that follows.
The Boeing Microbiology Lab identified the biomass as a fungus classified as Fusarium. Cultures may be brightly colored and are common in soil and dead or living plants, often causing plant disease.8 One of many clinical manifestations of Fusarium may include cutaneous and subcutaneous infections.
Because the second tank of a TEA etch rinse is not too alkaline, a commercial biocide could be considered. A biocide supplier10 was identified as having a product suitable to evaluate and their lab was willing to perform effectiveness testing. For our purposes, a kill dose was not necessary as long as there are no deleterious effects to parts, equipment and people from small (non-visible) quantities of the fungus. Thus, the objective was to focus on determining the minimum concentration of a biocide that would perform in the capacity of a biostat. The biocide supplier performed test media examinations, to determine which biocides to test. The significance of these tests is as follows11:
Measurement of pH: The pH of a test sample must be taken into consideration when selecting suitable biocides for the preservation of microbiologically susceptible products. Certain biocides remain more stable in products within a particular pH range than in others. Knowing the pH of a test sample is important in determining which biocides to test.
Measurement of Redox Potential: The Redox potential of a sample must be taken into consideration when selecting suitable biocides for the preservation of microbiologically susceptible products. Certain biocides remain more stable in products within a particular redox potential range than in others. Redox potential is a measure of the reducing or oxidizing capacity of a material. Measurement is determined using an appropriate redox electrode connected to a pH meter capable of reading the potential in mV.
Screening For Microbial Contamination: This test is used to determine the presence or absence of microorganisms in a sample and, if present, will determine the approximate degree of contamination. An exact count of microorganisms is determined using the Miles and Misra technique (below). This screening test is conducted prior to performing a wet state challenge test. A sample must be free from, or contain only a low level of, microorganisms in order to accurately assess the efficacy of a biocide. A biocide should not be added to a sample if the initial level of microbial contamination in the sample is too high, as this will cause some of the biocide to dissipate when added to the sample.
Microbiological Test Method12: This method is used to determine the total number of viable microorganisms in a sample—to quantify the microbial load. A known volume of a diluted sample is deposited onto the surface of a nutrient agar medium as drops from a calibrated pipette. After incubation, colony numbers are counted and a dilution factor is used to calculate total numbers of organisms or colony forming units (CFUs) in the original sample.
Microbial Kill Dose Test: This method is used to determine the concentration of biocide required to reduce the microbial contamination of an infected product to an acceptable level. After incubation for a suitable period, surviving organisms are detected by streak inoculation onto plates of nutrient growth media. The test may be used to determine a biocide “kill dose” for contaminated products. Additionally, samples in which the microbial population has been satisfactorily reduced may then be inoculated to determine long-term resistance by following the appropriate wet-state resistance method.
Wet-State Microbial Resistance Test: This method evaluates the anti-microbial resistance and determines the efficacy of biocides in water dilutable formulations. The method applies to aqueous dilutions of functional fluids. Functional fluids are defined as products that are supplied as concentrates, diluted with water and used in manufacturing systems under dynamic conditions, normally by pumping or re-circulating around appropriate manufacturing machinery. Some examples of functional fluids are metalworking fluids (lubricants and cleaners), and textile spin finishes. Biocides that may be used in such materials are tested by this method.
In order to determine the anti-microbial resistance of products and the efficacy of biocides that may be used in them, samples are prepared (normally by diluting with water) and agitated. They are challenged several times with an appropriate microbial inoculum, incubated, and monitored after each challenge.
A recommendation of 0.02% of the identified remedy biocide13 would be effective for the conditions of the subject process. Further discussion led to an agreement that an even lower concentration (0.015%) would be the proper concentration to qualify per BAC 5786.
Engineering tests were conducted to ensure that when Acticide GA is added to the second rinse tank of a TEA etch rinse, there will be no adverse affects to paint adhesion or corrosion resistance for anodized and conversion coated surfaces. Summarily, the tests conducted were: rivet chipping; room temperature impact; 100% relative humidity; 120°F tape test; wet/dry adhesion; corrosion resistance; and coating weight. Additionally, scanning electron micrographic (SEM) examinations were performed on the anodize structure to check structure morphology. All test panels processed were 2024-T3 bare. The paint system14 chosen was BMS 10-11 Type 1, Grade E and BMS 10-11 Type 2, Grade D. A total of 120 panels were produced for this qualification effort.
If implemented for the nine Boeing applicable rinse tanks, an estimated annual water savings of ~340,000 gallons would be realized. This translates to an estimated annual cost savings of ~$15,000.
Finally, Boeing Environmental Engineering required a determination of the amount of this biocide that would flow to the on-site waste treatment plant to assess ecological impact (Ref. 2). Using mass balance techniques, the following expression was derived15 for a double counter current (DCC) rinse tank:
CD(t) = FBCB/Ft + [FBCBFo/VCVD]/[(Fo/VD) - (Ft/VC)] {{-(VC/Ft)[exp(-Ftt/VC)]}+{(VD/Fo)[exp(-Fot/VD)]}}
where,
- FB= feed rate of biocide
- Ft= feed rate of process water into clean rinse
- FE1= evaporation from clean rinse
- FE2= evaporation from dirty rinse
- FW= dirty rinse sent to sump (prior to waste treatment)
- FO= overflow from clean rinse to dirty rinse = Ft– FE1
- CB= concentration of the biocide
- CD= concentration of the biocide in the dirty side
- VC= volume of the clean side
- VD= volume of the dirty side
- t = time
This equation can then be used for any size DCC rinse system and solved iteratively for time.
Boric-Sulfuric Acid Anodize
 Alternararia fungus culture.Bio-contamination of boric-sulfuric acid anodizing (BSAA) tanks was first noticed in a large 60,000-gal tank in late 1994 and later in other anodize tanks at the Boeing Auburn and Frederickson sites.
Alternararia fungus culture.Bio-contamination of boric-sulfuric acid anodizing (BSAA) tanks was first noticed in a large 60,000-gal tank in late 1994 and later in other anodize tanks at the Boeing Auburn and Frederickson sites.
The bio-mass was sampled and identified to be a fungus of the genus Alternaria. This fungus was prolific throughout the tank and mycelial fragments adhered easily to wing skin panels and wing spar components even after DCC rinsing.
This fungus is commonly isolated from decaying plant materials and also causes plant diseases. Spores of Alternaria species are dispersed by air currents and are usually a major component of outdoor air.
Due to the length of the aluminum airplane parts, manual spray rinsing was not feasible. Consequently, fungus dried to the part surface causing additional pre-paint cleaning. If undetected prior to painting, surface irregularities were easily seen after paint application and required costly rework to avoid certain in-service paint failures. The only near-term “fix” was to dump the process tank and steam-clean the tank interior. This was an unacceptable condition of lost process chemicals and—more importantly—a loss of valuable production operations due to three days of downtime. Additionally, the human impact of this fungal contamination was not as much a concern as the process chemicals themselves but could possibly result in conjunctivitis in extreme conditions.
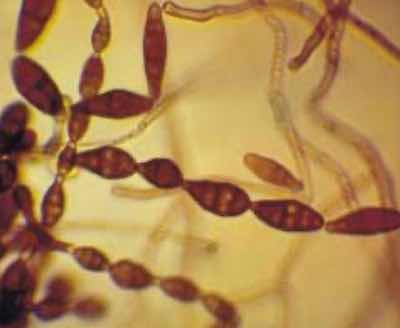 Alternaria mycelial fragments.Early efforts involved a “fall-out” plate testing of selected BSAA tanks at the Boeing Auburn site. Agar solution dishes were placed in various locations such as adjacent lip-boards, floor gratings and near the ventilation ducting. An analysis of results confirmed that each site had similar levels of ambient fungus that increases in quantity over time. This provided a correlation to the observation that uncovered tanks had more visible fungus contamination than tanks with lids.
Alternaria mycelial fragments.Early efforts involved a “fall-out” plate testing of selected BSAA tanks at the Boeing Auburn site. Agar solution dishes were placed in various locations such as adjacent lip-boards, floor gratings and near the ventilation ducting. An analysis of results confirmed that each site had similar levels of ambient fungus that increases in quantity over time. This provided a correlation to the observation that uncovered tanks had more visible fungus contamination than tanks with lids.
Early remedies discussed included: Allow higher Cu levels to inhibit the fungal growth; heat the process solution (temporarily); use filtration; identify a suitable fungicide; and install tank lids.
Each of these proposed options had certain drawbacks. Higher Cu levels would inhibit fungal growth but are limited by the BSAA process specification17 to be less than 155 ppm. The higher temperatures needed—in the range of ~160°F—would put the plastic fluid transport piping at serious risk. A filter size of ~0.45 microns would remove free-floating fungal contamination, but recontamination would quickly reoccur because the fungus adheres to the tank walls. Installation of tank lids would cause a grievous length of downtime and capital cost. And finally, identifying a suitable biocide would be difficult because commercial biocides are generally not stable in very acidic conditions (pH of <2). We know, however, that there is widespread use of benzoic acid and sorbic acid; these are commonly used as inhibitors in the food industry and elsewhere. This led to the decision to explore these chemicals as possible BSAA process solution additives because sodium benzoate is known to form a chemically stable complex with the aluminum ion (Ref. 3 and 4).
Subsequent effectiveness testing revealed that low levels of benzoic acid would indeed inhibit Alternaria growth in a BSAA solution. Consequently, an effort to perform engineering testing (qualification program) was initiated in 1996. The test program was rather extensive involving determination of the effects of benzoic acid to the BSAA formulation. The tests conducted were:
- Corrosion resistance per ASTM B117 (336 hour salt spray)
- Wet/dry paint adhesion
- Scanning electron microscopy (SEM) examination of coating
- Coating weight
- Percent seal hydration
- Primer/topcoat rivet chipping
- Room temperature primer impact
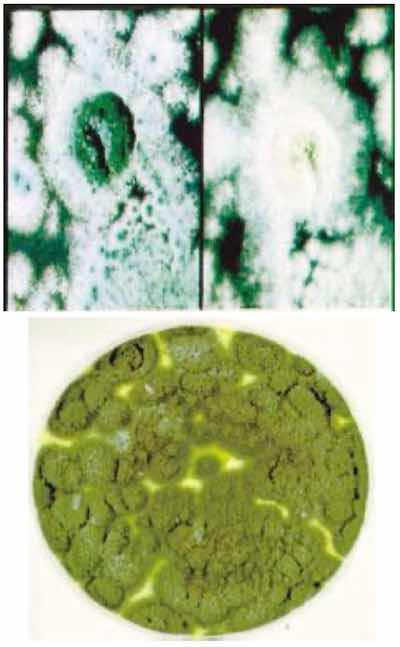 Penicillium fungus cultures.The analysis was conducted relative to a BSAA control solution at high and low boric acid concentration and mid-range for the sulfuric acid concentration. Successful completion of this testing led to the inclusion of benzoic acid in the BSAA process specification.18 However, the solid benzoic acid flakes are only slightly soluble at bath temperature (0.2% by weight). Thus, the add procedure required benzoic acid to be dissolved in warm water at a concentration less than 2 g/L. The concentrated solution could then be added to the bath as part of make-up water or as part of the initial bath charge. Processing would not be allowed if undissolved flakes were visible on the solution surface. An improved procedure was to allow the use of a benzoic acid salt (sodium benzoate), which could be added directly to the BSAA tank, enabling rapid dissolution. The resulting addition of sodium to the BSAA solution is estimated to be ~50 ppm after three fungicide adds of 100 ppm each. Because 100 ppm of chloride (measured as sodium chloride) is allowed, this amount of sodium should be no cause for concern.
Penicillium fungus cultures.The analysis was conducted relative to a BSAA control solution at high and low boric acid concentration and mid-range for the sulfuric acid concentration. Successful completion of this testing led to the inclusion of benzoic acid in the BSAA process specification.18 However, the solid benzoic acid flakes are only slightly soluble at bath temperature (0.2% by weight). Thus, the add procedure required benzoic acid to be dissolved in warm water at a concentration less than 2 g/L. The concentrated solution could then be added to the bath as part of make-up water or as part of the initial bath charge. Processing would not be allowed if undissolved flakes were visible on the solution surface. An improved procedure was to allow the use of a benzoic acid salt (sodium benzoate), which could be added directly to the BSAA tank, enabling rapid dissolution. The resulting addition of sodium to the BSAA solution is estimated to be ~50 ppm after three fungicide adds of 100 ppm each. Because 100 ppm of chloride (measured as sodium chloride) is allowed, this amount of sodium should be no cause for concern.
In BSAA solutions, the test method to determine the concentration of the biocide involves spiking a bath sample at several concentration levels with sodium benzoate and then measuring the UV absorbance of each solution. The original concentration can then be determined by the method of standard additions.
Factory trials for this improved BSAA solution were performed in the largest anodizing tank at Boeing (65,000 gallons). The trials were performed in this production tank, which had fungal contamination levels of about 20 colony forming units per milliliter (fungus of the genus Alternaria). These trials demonstrated the effectiveness of the fungistat and resulted in no significant change of coating weight and did not affect salt spray results for the improved BSAA process in actual production use. As a preferred practice, the fungistat is added to a newly prepared BSAA bath that has no fungus present, so it may serve to prevent or inhibit fungal growth. The addition of the fungistat will not necessarily remove or dissolve any fungal biomass already present in a BSAA solution, but it will prevent or inhibit any further growth. An implementation that followed at the Boeing Frederickson, WA site led to the realization that the allowable concentration could be increased to 1,000 ppm and still have no adverse effects on the 7000-series aluminum large wing parts being processed there.
As evidence of the importance of this effort, the Boeing Company pursued and obtained a U.S. patent entitled “Fungus Resistant Boric Acid – Sulfuric Acid Anodizing”19 as an improvement to the patent issued January 16, 1990 entitled “Method for Anodizing Aluminum.”20
Boric-Sulfuric Acid Anodize Rinse
Further evidence as to the importance of thorough bio-organism identification is the case of BSAA rinsing where the observed contaminant was not Alternaria but another species of fungus. Contaminants were observed sticking to the aluminum parts after the first rinse following anodizing. This first rinse of a BSAA double counter-current (DCC) rinse system can be fairly acidic (pH < 2).
This rinse was sampled and was found to be inhabited by a fungus (Penicillium). Many species of Penicillium are common contaminants on various substrates and are known as potential mycotoxin producers. Human pathogenic species are rare, however, opportunistic infections can lead to mycotic keratitis.21 The most common human diseases caused by this class of fungi are infections of the skin and mucous membranes, such as ringworm and thrush.22
Efficacy testing, with sodium benzoate, concluded that 500 ppm would be a suitable biocide concentration to inhibit fungal growth.
Engineering testing included: a) rivet installation; forward impact testing24; and epoxy primer25 paint adhesion26. Sodium Benzoate was qualified (3/28/2002) as a rinsewater additive for BSAA solutions per a Process Specification Departure (PSD 6-53) to the Boeing process specification, BAC 5632.
Acid Etch Rinse
The first rinse of a DCC rinse system that follows a nitric acidetch was found to have bio-contamination that adhered to the parts even after air-agitated immersion rinsing. The bio-contamination was identified to be a plethora of bacteria (Ref. 6) and fungus (Ref. 3). The most dominant of these were bacterial species of Pseudomonas and Acinetobacter.
Because, this rinse is fairly acidic, commercial biocides would again have difficulty maintaining stability. This acid rinse had numerous bacterial (Pseudomonas, Acinetobacter, Xanthomonas) and at least one fungal colony (Exophiala). Because the numbers of species were so prolific and this acid-etch rinse is lower in pH (<2) than is recommended for most commercial biocides, a potential remedy was to evaluate peroxyacetic acid (PAA). This chemical has common use in the food industry as a disinfectant and became of particular interest in that “overuse and misuse of traditional biocides leave an open field for opportunistic bacteria that would otherwise be kept in check by other bacteria” (Ref. 5). Additionally, aluminum is the only common metal that does not catalyze the decomposition of hydrogen peroxide (Ref. 6). Peroxyacetic acid is rapidly cidal at low concentrations and its decomposition products (acetic acid, water and oxygen) are innocuous and environmentally acceptable (Ref. 7).
After successful efficacy testing, engineering requirements were established as salt spray testing for the following alloys: 300 series SS; 400 series SS; 15-5 PH; and Ti-6Al-4V for qualification to Boeing process specification, “Surface Treatment of Ferrous Alloys” 27 and “Cleaning, Descaling and Surface Preparation of Titanium and Titanium Alloys”.28 This is consistent with the Little, Ray and Wagner recommendation of using oxidizing biocides not in the process solution but rather in the rinse tanks (Ref. 8). Recently (4/4/2003), PAA was approved for in-process use in rinse tanks of the aforementioned acid etch processes.
Additional uses of PAA (peroxyacetic acid) have been realized as a “clean-out” chemical to add to rinse tanks before they are dumped to the on-site waste treatment plant. Thus far, a conversion coating rinse and a heat treat quench rinse have been treated with PAA prior to dumping, and results have been promising in that dump frequencies have decreased. Had this effort been unsuccessful, the fall-back plan was to evaluate 2-(Decylthio)ethanamine Hydrochloride (Ref. 9).
Corrosion
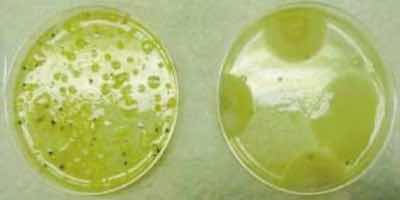 Acid-etch rinse cultures.Microbially induced corrosion (MIC) is a topic well documented. Corrosion of auxiliary equipment can occur as a result of slime formation. Choudhary reports that the buildup of biological slime can occur on heat-transfer surfaces in as little as 4 to 8 hours (Ref. 10). The basic mechanism theories vary (Refs. 11, 12, 13) but basically involve the removal of certain major or minor metallic atoms from the structure of the alloy by extracelluar enzyme activity. Other mechanistic theory involves the created imbalance of ionic compounds. Blanchard and Goucher believe that microorganisms remove phosphate and nitrate more rapidly than calcium or iron from the media in which they grow (Ref. 14). At Boeing, recent research and development efforts29were expended to provide a usable test method that measures a process solution’s tendency to cause pitting. Certain measures, such as “the critical pitting potential ECPP, is usually defined as the most noble potential at which the passive current density remains stable and pits do not nucleate on a crevice-free surface. This value is measured potentiostatically in the laboratory under carefully controlled conditions. If measured carefully, the ECPP, is independent of the geometry of the specimen and the test apparatus. In contrast is the breakdown potential Eb, defined as the potential at which the anodic polarization curve shows a marked increase in current, leading to breakdown of the passive film and pit initiation” (Ref. 15). The closer EPIT is to ECORR, the greater the probability that pitting will occur (Ref. 16) in the process solution. These techniques were conducted by several researchers and, in part, they revealed that MIC attack is most susceptible on 2024 Al alloy and least susceptible on clad aluminum (Ref. 17).
Acid-etch rinse cultures.Microbially induced corrosion (MIC) is a topic well documented. Corrosion of auxiliary equipment can occur as a result of slime formation. Choudhary reports that the buildup of biological slime can occur on heat-transfer surfaces in as little as 4 to 8 hours (Ref. 10). The basic mechanism theories vary (Refs. 11, 12, 13) but basically involve the removal of certain major or minor metallic atoms from the structure of the alloy by extracelluar enzyme activity. Other mechanistic theory involves the created imbalance of ionic compounds. Blanchard and Goucher believe that microorganisms remove phosphate and nitrate more rapidly than calcium or iron from the media in which they grow (Ref. 14). At Boeing, recent research and development efforts29were expended to provide a usable test method that measures a process solution’s tendency to cause pitting. Certain measures, such as “the critical pitting potential ECPP, is usually defined as the most noble potential at which the passive current density remains stable and pits do not nucleate on a crevice-free surface. This value is measured potentiostatically in the laboratory under carefully controlled conditions. If measured carefully, the ECPP, is independent of the geometry of the specimen and the test apparatus. In contrast is the breakdown potential Eb, defined as the potential at which the anodic polarization curve shows a marked increase in current, leading to breakdown of the passive film and pit initiation” (Ref. 15). The closer EPIT is to ECORR, the greater the probability that pitting will occur (Ref. 16) in the process solution. These techniques were conducted by several researchers and, in part, they revealed that MIC attack is most susceptible on 2024 Al alloy and least susceptible on clad aluminum (Ref. 17).
Bio-contaminants are usually detected in the process solution but can remain undetected and remain dormant on part surfaces until the airplane parts are in service. There is an abundance of literature on problems associated with fuel tank corrosion (Ref. 13, 18, 19).
Summary
Bio-contamination in metal finishing tank-line operations is a common problem, occurring in both process solution and rinse tanks, across wide pH ranges. Several biocides, bio-stats and disinfectants have been shown to be effective in destroying bio-organism growth or limiting its growth capability. Lessons from the food industry have led to the use of generic chemicals (PAA and sodium benzoate) as process solution and rinse water additives. The advantage of using these additives is predominantly a reduction in downtime, but their use also provides a modest reduction in water use, and the potential prevention of human infection and equipment corrosion.
References
- Corrosion of Aluminum and Aluminum Alloys, J.R. Davis & Associates, 1999.
- Electrochemical Monitoring of Aerobic Bacteria and Automation of Biocide Treatments, L. Guiuliani, 1996.
- Chemical Treatment of Aluminum Alloys with Sodium Oxalate Solution, Isobe, Tanaka & Hine, October 1990.
- Water Treatment, website: http://www.aluinfo.dk/Html/alulib/ modul/A00200.htm.
- War on Bacteria Could Leave Drug-Resistant Strains Unchecked, American Society for Microbiology, 7/19/2000.
- Corrosion Studies of Aluminum in Chemical Process Operations, Cook, Horst and Binger, 1/16/1961.
- CORROSION 97, Peracetic Acid: A New Biocide for Industrial Water Applications, Kramer, 1997.
- Tame Micribiologically Influenced Corrosion, Little, Ray, & Wagner, 1998.
- CORROSION 97, 2-(Decylthio)ethanamine Hydrochloride: A New Multifunctional Biocide Which Enhances Corrosion Inhibition, Walter & Cooke, 1997.
- Emerging Microbial Control Issues in Cooling Water Systems, Choudhary, May 1998.
- The Nature of Sulfide Compounds and Their Formation, Allen-Murray Corp.
- Microbiological Corrosion of Aluminum Alloys, Hedrick, Applied Research Laboratory.
- Mechanism of Microbiological Contamination of Jet Fuel and Development of Techniques for Detection of Microbiological Contamination, Part I, January 1964 .
- The Corrosion of Aluminum by Microbial Cultures, Blanchard & Goucher, 1964.
- Use and Limitations of Electrochemical Techniques for Investigating Microbiological Corrosion, Dexter, Duquette, Siebert, Videla, April 1991.
- Microbiologically Influenced Corrosion of Metals and Alloys, Little, Wagner, and Mansfield, 1991.
- Microbial Induction of Corrosion in Aircraft Aluminum Alloy Sheet, Landerkin, Cheminant, 1968.
- Microbiological Fuel Tank Corrosion: mechanisms and contributory factors, Churchill, 1962.
- Mechanism of Microbiological Contamination of Jet Fuel and Development of Techniques for Detection of Microbiological Contamination, Part II, February 1966
About the Author
W. John Fullen is with Manufacturing Research & Development, The Boeing Company, Seattle, WA. He can be reached at 253-218-8710; email: warren.j.fullen@boeing.com.