Water-based chemistries along with electrical power are used to create a coating made primarily of aluminum oxide. The coating actually “grows” out of the metal making the hardness and wear resistance an order of magnitude greater than paint, and unlike paint, both the process and the coating are considered eco- friendly.
 Mark JozefowiczDesigners often use descriptive terms like “young” and “quality” when describing an anodize finish. It is the material of choice for a vast array of applications ranging from architectural to decorative to functional. This multi- step finishing process begins with chemical preparation of the aluminum, followed by anodizing, possibly coloring, and finally sealing.
Mark JozefowiczDesigners often use descriptive terms like “young” and “quality” when describing an anodize finish. It is the material of choice for a vast array of applications ranging from architectural to decorative to functional. This multi- step finishing process begins with chemical preparation of the aluminum, followed by anodizing, possibly coloring, and finally sealing.
Specifications
The most commonly used are the military MIL 8625F or its derivatives, and the architectural AAMA 611. The former discusses Type I (chromic acid anodizing), Type II (normal sulfuric acid anodizing), and Type III (hard coat anodizing). Sub classifications include Class 1 (clear) and Class 2 (colored). Similarly, the architectural specification discusses Class 1 & Class 2 finishes, however in this case the reference is made to thicker & thinner (more/less durable) sulfuric acid anodized coatings. Both specifications refer to tests used to quantify durability requirements.
Effect Of The Aluminum Alloy
Essentially all aluminum is alloyed for varying effect. Typical additions include copper, magnesium, silicon, zinc, and manganese. Aluminum can be made stiff, ductile, and corrosion resistant through the addition and manipulation of these foreign elements. Because the anodize coating “grows” out of the metal, anything in the metal is included in the coating. Alloying elements can have negative finishing consequences and often limit the applicability of the type of finish available.
Effect Of The Metal Forming Process
Aluminum can be shaped by methods that include casting, extrusion, forging and machining; each with its own special alloy requirements. If not done properly, the forming process can affect the outcome of the finished part. Castings may have white spots, extrusions may be blistered or have longitudinal bands, machined parts may be pitted. The finisher may be able to adjust the process to mitigate many of these defects, but this is not always the case.
Considerations For Part Design
Dimensional change consideration: During anodizing, aluminum is converted to aluminum oxide. Surface aluminum is consumed as oxide is created with net dimensional gain. The general rule for conventional “Class II” coatings is 2⁄3 in 1⁄3 out. This can be explained by example: If a coating is specified to be 12 μm in thickness, 8 μm of aluminum will be consumed and 12 μm of coating will be created, for an overall net dimensional gain of 4 μm. The general rule for hard coat “Class III” finishes is 1⁄2 in 1⁄2 out. For close tolerance parts, this dimensional change must be considered in the design phase.
Contact points: Being an electro-chemical process, electrical contact must be made between the part and the rack fixture. A defect will occur at the point of contact, so they should be made in out of the way locations. Contacts are often made on an edge of the part or on the inside of a hole. The number of necessary contact points will depend on the part size as well as its geometry. A discussion of contact point location should occur between the anodizer and the part manufacturer.
Process Flow
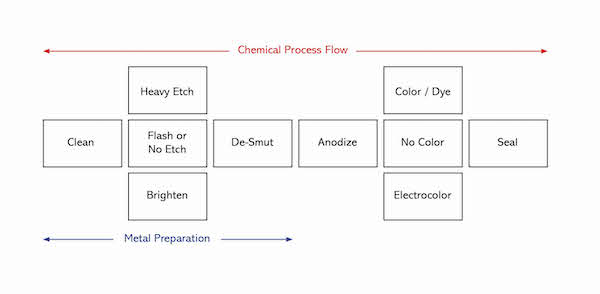
A schematic illustration of the anodizing process is shown below.
Metal Preparation
Clean: Clean metal is needed for a quality finish. Soils must be removed and contained within the cleaner. Cleaners are a synergistic blend of builders, surfactants, and chelators that usually perform best at 120°F-160°F and with agitation. Some are acidic but most are alkaline as these tend to be “heavy Duty”. Cleaners should not be overused. A general rule is to change it out when additions total the amount of the initial charge.
Etch: A flash etch of one minute or less is often used to prepare the surface for anodizing. A heavy etch will impart a low gloss luster to the aluminum surface, and is therefore used for its decorative appeal. Etch chemistries are typically based on either caustic soda or, more recently, ammonium bifluoride. In both cases, the active chemical attacks the aluminum and creates micro pits on the surface. The pitted surface is responsible for scattering light, thereby lowering the apparent gloss. The chemical reaction is vigorous and requires careful control to ensure an optimum chemical balance. Bath analysis must be carried out at regular intervals, and is done using simple titrations.
Brighten: To impart a high gloss finish, a brightening step is used instead of etch. This process may, or may not include electrical stimulation. The active components of the more common non-electrical process are nitric acid (to convert the aluminum to its oxide), and phosphoric acid (to dissolve the formed aluminum oxide). These two opposing reactions take place simultaneously in a highly concentrated acid environment at very high temperature. The result is a leveling of the aluminum surface. Brightening is best done on special alloys of aluminum with very low iron content
De-smut: A by-product of both etch and brightening processes is a residue that is left on the surface of the aluminum. The de-smut simply clears these unwanted materials to prepare the aluminum for anodizing.
Anodize
To anodize aluminum is to oxidize it in a controlled environment using an acid bath and electrical energy. While submersed, the aluminum is connected to the positive terminal of DC power supply and thus made the anode. Counter electrodes are connected to the cathode. The bath is normally sulfuric acid. When the power is turned on, electrical current flows and a porous aluminum oxide coating begins to “grow” on the anode (hence “anodize”). Coating characteristics (thickness, porosity, color) can be controlled when attention is paid to the following:
Chemistry: Conventional (Type II) anodize requires the use of a sulfuric acid bath that contains 160-200 g/l acid and 2-15 g/l dissolved aluminum. Operational temperature is critical and should be maintained at 70 +/- 2 ◦F. Since this process generates heat, a refrigeration unit and cooling coil must be designed correctly and agitation employed. Hard coat (Type III) coatings are anodized under accelerated conditions with lowered bath temperature. Since this treatment is much more aggressive, particularly close attention to process parameters is required.
Power: For conventional (Type II) anodize, the voltage is raised within 1 minute to approximately 18 volts to achieve a “current density” of 15 Amps for every square foot of load area. Coating growth rate is proportional to current density therefore monitoring electrical current is preferred over voltage as the latter can change with alloy, bath conductivity and load size. This is especially true for hard Coat (Type III) anodizing where the process is accelerated and there is little room for error. Hard coat current densities are often twice as high as conventional or more, and the voltage may increase during the process from 20V to 80V.
Alloy: Every alloy reacts differently in the anodize bath, but none more so than aluminum alloyed with copper. Coatings on copper alloys grow at a slower rate and reach their limit thickness far before other wrought alloys. Special consideration should be given to these alloys by the finisher as it is often the case in specifications. The natural color of the coating is also dependant on the alloy. Silicon alloys are grey; zinc alloys can have a yellow tint. High silicon castings are non-uniform in composition and can, as a result, be dark grey with unsightly surface flow patterns.
Time: Under standard (Type II) anodizing conditions (alloy, temperature, and current density), processing time will govern the coating thickness. A well practiced rule is to anodize five minutes for every 0.0001 inch (0.1 mil) target thickness. A 0.4 mil coating should take 4 x 5 = 20 minutes.
Color
An anodized coating is porous and as such it can be colored. The most commonly used coloring technologies are electro-color (also called 2-Step) and dye. Electro- color is usually associated with architectural applications since the various shades of bronze to black are light fast. Immersion dyeing can produce a myriad of colors but they are generally not suitable for long-term outdoor exposure.
Electro-color: Commercial buildings will often have an aluminum/glass curtain wall and fenestration colored either bronze or black. As its name implies this coloring technique uses electricity. AC power is applied to the anodized part while it is submerged in a solution containing a dissolved metal (usually tin). After a brief soak, voltage is ramped to about 18V and then held for a set period of time. During this time particles of tin are deposited inside the pores of the coating creating a color. Over time the color deepens from very light bronze to medium, dark, and eventually black.
Immersion Dye: A dye bath is made by dissolving specialized dyes in de-ionized water, adjusting the pH, and in most cases heating to 140°F. Anodized parts are suspended in the dye bath and allowed to soak up the color. The longer the soak and the more concentrated the dye, the deeper the shade. Dyes can be used alone or in combination, opening up a realm of color possibilities. All dyes fade to some extent when exposed to sunlight, but some have been found to be more stable than others. The Sanodal system describes how these dyes can even be used to create fade resistant architectural quality aluminum finishes.
Seal
By sealing the porous anodize coating, color is locked in and corrosive elements are locked out. Depending on the type of seal used, the pores within the anodize coating are closed by hydration (swelling of the pore walls), impregnation, or both. Seal chemistries are generally classified as nickel based or nickel free. Some run hot while others are used at room temperature. Each type of seal has its positive as well as negative attributes but all have their own specific requirements for immersion duration, bath temperature, pH, chemical content, and maximum level of contaminants. The latter primarily being phosphates and silicates.
Recommendations, notices or instructions as to handling, use, storage or disposal of this product, including its use alone or in combination with other products, or as to any apparatus or process for its use are based upon information believed to be reliable. No liability is taken with respect to any such recommendations or instructions. Sole and exclusive warranty is that products comply with published chemical and physical specifications as provided on the certificate of analysis. No other warranties, either express or implied are given.
Mark Jozefowicz is Vice President of Technical Services at Reliant Aluminum. Visit http://www.reliantaluminumproducts.com



































