The acid etch referred to in this technical data sheet is the process that North American architectural anodizers have been adopting since 2005.
 Mark JozefowiczThe chemistry is based on ammonium bifluoride combined with adjuncts to improve finish quality and reduce chemical consumption.
Mark JozefowiczThe chemistry is based on ammonium bifluoride combined with adjuncts to improve finish quality and reduce chemical consumption.
In general, etching aluminum provides several different features
- Removes ground in impurities
- Smooth out surface imperfections such as extrusion lines and mild scratches
- Removes ground in oxide and scale
- Produces a uniform smooth surface
- Changes the natural brightness to a dull matte finish
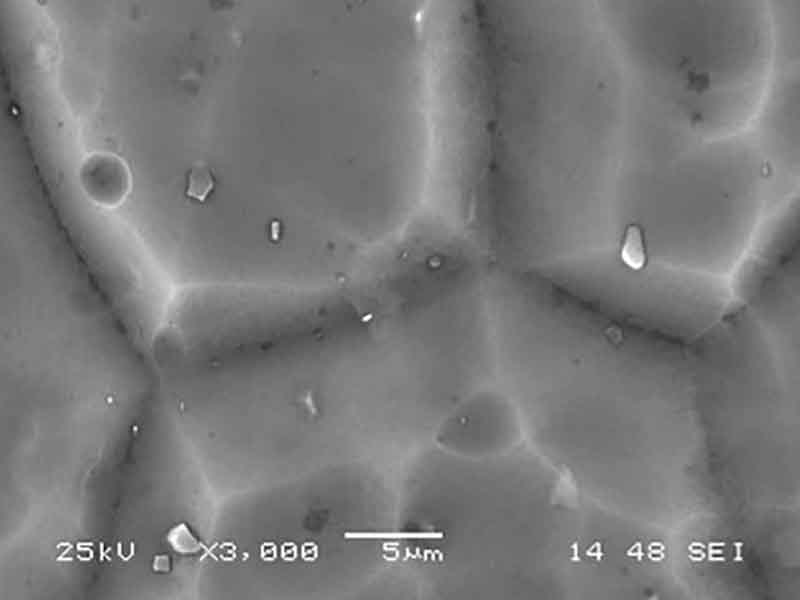
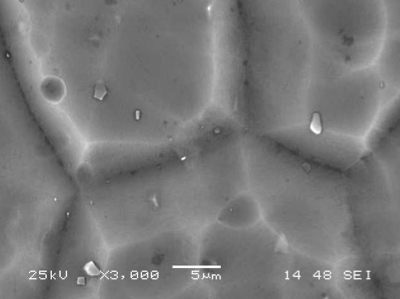
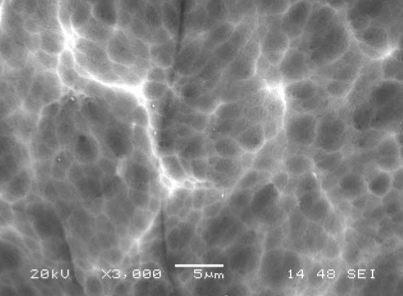
A conventional caustic etch creates relatively large pits that tend to follow aluminum grain boundaries, imperfections and inter-metallic particulates. It is a coarse etch that, by eating away enough aluminum reduces the gloss level to an acceptable value. To the contrary, an acid etch will produce a higher population of smaller, evenly dispersed pits, consuming far less aluminum. The difference in the appearance of the surface is depicted in the SEM pictures below:
 Caustic Etched 6063 T-5 Panel Caustic Etched 6063 T-5 Panel |
 Acid Etched 6063 T-5 Panel Acid Etched 6063 T-5 Panel |
Benefits
Acid Etch produces a superior low gloss matte finish for hiding metal imperfections such as die lines, extrusion welds, and large grain “spangle”. It enables the anodizer to successfully process metal that could have been originally deemed as scrap and it improves line efficiency by reducing re-work. Compared to conventional caustic etch, 80-90% less aluminum needs to be removed from the surface to achieve the desired gloss. As a result, aluminum sludge volume is lessened, finished aluminum (selling) weight is increased, and rack life is improved.
Chemistry and Consumption
Ammonium bifluoride (ABF) reacts with aluminum (etches) to produce ammonium hexafluoroaluminate and hydrogen gas. The pH of an acid etch is usually maintained at or near 5.0. The temperature is kept near 115 ̊F, and the time, usually 2-5 minutes. The chemical reaction that occurs is as follows:
3NH4FHF + Al → (NH4)3AlF6 + 1½ H2
Based on this equation, 27g aluminum reacts with 171g ABF and produces 195g sludge (dry weight). Considering the optimum weight loss for acid etched aluminum is 1.0-1.5 g/ft2, 1 lb of ABF is theoretically needed to etch 60 +/- 12 ft2 aluminum which in turn will produce about 1.1 lb sludge (dry weight). Very little heat is generated by the reaction, so as a result, cooling of the bath is not generally needed.
Since the reaction product is insoluble, it immediately precipitates out of solution. When the agitation is turned off, the solids settle quickly and compactly. Solids are generally monitored by sampling the agitated bath and allowing them to settle in a graduated cylinder. A volume measurement is taken. The level of solids in the bath should be kept as low as possible and never allowed to exceed 10% by volume. ABF concentration is best measured by selective ion electrode, in this case fluoride.
Process and Production
With respect to etch defects, the acid etch is a relatively forgiving process. The few problems that are encountered are usually due to the acid content being too low (staining), or suspended solids being too high (pimpling). Since the bath is not viscous, there is no problem with rinsing, and because the etch period is generally only a few short minutes, production bottlenecks do not occur.
The finish produced by a stand-alone acid etch is generally too “chalky” in appearance, so in practice it is usually followed by a very brief (0.5-2 min) conventional caustic etch. The caustic etch brings back the natural metallic look that one associates with anodized aluminum.
Below is an example of the pretreatment portion of an anodize line set up for acid etching.
- Clean
- Rinse
- Acid Etch (Times may vary from 2 to 5 minutes)
- Rinse
- Caustic Etch (Determined by desired finish)
- Rinse
- Rinse
- Deoxidize
- Rinse
Materials and Equipment
Due to the highly corrosive nature of acid fluoride solutions, metal in any form should not be designed to come into long-term contact with an acid etch. It is preferred that either plastic or plastic lining be used for process tanks, pumps, piping etc. The heating source, preferably PTFE coated, should be designed to achieve a tank temperature of 120 ̊F. Other requirements include a filter press and an exhaust system with scrubber.
Environmental Health and Safety
As with any chemical used on an anodize line, appropriate PPE including gloves, boots, apron and face shield should be used by individuals handling or otherwise potentially coming into bodily contact with acid etch. Due to the unusually large volume of chemical additions required to keep the chemistry within acceptable limits, additions made by hand directly to the tank can be tedious, time consuming and sometimes dangerous. As a result, a pumping system is generally set up between a premixing station and the tank.
The pH of post etch rinse tanks and waste treatment neutralization systems, if allowed to rise too high will result in the release of noxious ammonia fumes. Appropriate precautions should be taken.
The sludge generated by the process, although greatly reduced when compared to a conventional caustic etch process, must be removed and disposed of in a manner consistent with local and state agencies. Direct dischargers should also consider that nitrogen levels in their waste will be elevated and that equipment for remediation may be required by the local governing agencies.
Mark Jozefowicz is Vice President of Technical Services at Reliant Aluminum. Visit http://www.reliantaluminumproducts.com