For many high precision industries such as aerospace, automotive or medical technology, industrial parts cleaning plays a crucial role in quality assurance during the production process.
 Richard StarkeyInadequate degreasing can have a disruptive effect on many subsequent processing steps. Nevertheless, we see far too many companies using a suboptimal cleaning solution in their operations.
Richard StarkeyInadequate degreasing can have a disruptive effect on many subsequent processing steps. Nevertheless, we see far too many companies using a suboptimal cleaning solution in their operations.
Some businesses simply lack the knowledge to competently evaluate potential options. Others have been relying on one particular method for so long that they are not aware of alternative solutions. And in many cases, deep-seated misconceptions about a certain cleaning technology are leading to a categorical ruling out of their usage.
A case in point is solvent cleaning. Many manufacturers are coming under significant corporate pressure to move away from solvents to water-based cleaning in the name of achieving greater safety and sustainability. What is troubling is that sweeping decisions are often made based on a distorted view on how solvent and water cleaning work.
The truth is every cleaning system has its own environmental impact that manifests in different ways. Whether it is solvent or water cleaning, both processes require prudence and diligence from users in ensuring worker safety, environmental protection and regulatory compliance. Making an informed decision means evaluating all facts, data and key aspects objectively. And this must first start with overcoming preconceived notions.
Cleaning Effectiveness – Is One Better Than The Other?
Both solvent and water cleaning are effective cleaning media, but the evaluation of their use must be tied to the specific context. Water-based cleaning can be an excellent choice for polar contaminations, like salt residues. The process can be more efficient when working wet in wet (e.g. water-based painting, galvanisation). Depending on the pH-values, it does not impact rubbers, plastics, or painted surfaces. Cleaning can also be combined with surface finishes such as phosphating, chromating or deposition of protective coatings. Because of the different formulations available, water cleaning can also be adapted to specific cleaning requirements.
Solvents on the other hand are first choice for cleaning off non-polar contaminants, like oils and greases. It also has universal compatibility with metals (please note that water-cleaning agents can be acidic, neutral or alkaline and are usually matched to specific metal types). Because of their good creeping capability, solvents are particularly suited to cleaning complex geometries or tiny parts.
What is the Environmental Impact?
Many people tend to associate water with “being green”. However, water alone cannot clean off contaminations entirely. Most water-based cleaners contain surfactants, biocides, complexing agents, dyes, fragrances etc. Some of these compounds are not biologically degradable and, depending on their concentration, may be harmful to fauna and flora in surface waters.
Not to forget that water itself is a finite resource. Water-based cleaning also comes with significant energy demand. There is the requirement to operate high-pressure pumps, heat the cleaning water, dry the metal parts, as well as treat and purify used water for re-use or disposal. As a relatively slow-drying cleaner, water requires 10 times more of the latent heat of vaporisation (2259 J/g) than that of solvents (200-300 J/g).
 Solvents in contrast have good drying behavior and can penetrate easily into tight spaces. Of course, such properties can also bring inherent risks such as air emissions or ground penetration. The use of modern, closed machine technology, in combination with a closed loop solvent delivery system, is therefore key to enabling an emission-free and spill-free transport, storage and handling of solvents.
Solvents in contrast have good drying behavior and can penetrate easily into tight spaces. Of course, such properties can also bring inherent risks such as air emissions or ground penetration. The use of modern, closed machine technology, in combination with a closed loop solvent delivery system, is therefore key to enabling an emission-free and spill-free transport, storage and handling of solvents.
Solvent cleaning does not require heaters or blowers since parts readily come out dry from the cleaning machine due to the vacuum condition. Through built-in distillation in closed cleaning machines, solvents can be continuously recycled and there is less waste generated. The most modern solvent systems with up-to-date solvent management practices can remove 100 kg of oil using less than 15 kg of solvent . In comparison, to clean off 100 kg of oil, approx. 100 kg of water-based cleaner is required in a water-based system.
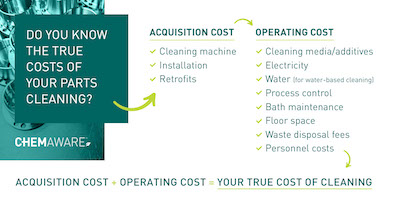
What is the Cost of Ownership?
The true cost of ownership must take into account – in addition to the acquisition cost – long-term operating expenses such as chemicals, utility bills, floor space, and waste disposal charges.
Standard solvent machine technology and initial first fill chemicals often require a higher capital investment than water-based cleaning. But solvents can be continuously reused and recycled via the machine’s distillation unit. There are thus much lower solvent replacement volumes and fewer bath exchanges necessary. In aqueous cleaning, since dirts and soils are emulsified and rinsed off, aqueous baths that are not treated will have to be replaced frequently.
Another key cost factor is labor time for operation and process monitoring. In solvent cleaning, only stabilisers are added (when necessary), so the chemistry in the vapor degreaser remains relatively consistent and requires little attention to ensure a constant cleaning performance. In water cleaning, various chemicals such as builder and surfactants are added to make the cleaning of non-polar contaminations possible, their concentration therefore requires consistent monitoring and adjustments.
The Bottom Line …
Every cleaning process comes with its unique requirements and challenges. By no means can solvent be a one-size-fits-all solution. However, companies would be doing themselves a huge disservice if they immediately rule out solvent cleaning without taking an objective evaluation. More than a necessity, parts cleaning can contribute to operational efficiency, resource preservation as well as sustainability – but it all hinges on making the right informed decision.
Richard Starkey is the UK Industry Manager at SAFECHEM. Visit www.safechem.com



































