Manufacturers are exploring critical cleaning processes that use a modified alcohol or an iso-paraffinic blend.
 Barbara and Ed Kanegsberg, and Professor Darren Williams.There are a number of reasons. Some manufacturers find that aqueous cleaning does not provide the appropriate cleaning efficacy. Many other manufacturers are adjusting to the reality that many effective cleaning agents are under impending regulatory activity, notably, EPA determinations that methylene chloride, trichloroethylene, perchloroethylene, and n-propyl bromide pose “unreasonable risks to workers.”
Barbara and Ed Kanegsberg, and Professor Darren Williams.There are a number of reasons. Some manufacturers find that aqueous cleaning does not provide the appropriate cleaning efficacy. Many other manufacturers are adjusting to the reality that many effective cleaning agents are under impending regulatory activity, notably, EPA determinations that methylene chloride, trichloroethylene, perchloroethylene, and n-propyl bromide pose “unreasonable risks to workers.”
As a result, some well-understood cleaning solvents are in short supply. It may be time to try something different. Different, in the world of molecules, means different structures. We continue to explain chemical structure because molecular structure determines function, including cleaning performance. Read on to get real about the benefits, considerations, and limitations of two important cleaning agents.
We introduced chemical structure in Part I of this series. We suggest that you review Part 1. (Ref. 1)
Isoparaffins (Paraffin Hydrocarbons)
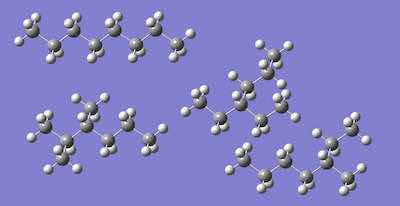 Figure 1. n-octane, three isomers of octane.Isoparaffins are petroleum distillates. They are mixtures of branched medium-chain alkanes. The term “paraffin hydrocarbons” is also used. To provide perspective, isoparaffins blend to have a higher boiling point than trichloroethylene and water. They are mixtures of non-polar organic solvents. Isoparaffins vary; as a mixture, it is not a single molecule.
Figure 1. n-octane, three isomers of octane.Isoparaffins are petroleum distillates. They are mixtures of branched medium-chain alkanes. The term “paraffin hydrocarbons” is also used. To provide perspective, isoparaffins blend to have a higher boiling point than trichloroethylene and water. They are mixtures of non-polar organic solvents. Isoparaffins vary; as a mixture, it is not a single molecule.
Rather than listing the formula, it is more informative to describe the molecular structure using “ball and stick” models. In Figures 1 and 2, carbon is shown as a grey ball; hydrogen is white; every single co-valent bond holding the elements together in the molecule is depicted as a thin stick that that looks somewhat like a toothpick.
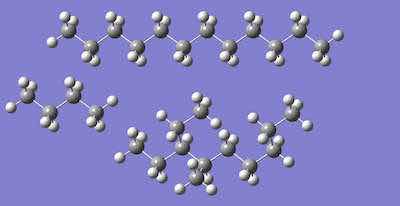 Figure 2. n-dodecane (top), Butane (middle), and one iso-dodecane (there are 355 isomers).High octane gasoline is a petroleum distillate. Figure 1 depicts n-octane (8 carbons in a row) and three isomers of octane (Ref 2). “High octane” refers to fuel of high quality and is related to the isomers of n-octane in the fuel. There are 18 structural isomers of octane, more including stereoisomers. Stereoisomers differ in the arrangement of atoms rather than the order of connection of atoms. For example, optical isomers have the same connections of atoms, but they are mirror images of each other – just like your two hands with the same bone structure are mirror images.
Figure 2. n-dodecane (top), Butane (middle), and one iso-dodecane (there are 355 isomers).High octane gasoline is a petroleum distillate. Figure 1 depicts n-octane (8 carbons in a row) and three isomers of octane (Ref 2). “High octane” refers to fuel of high quality and is related to the isomers of n-octane in the fuel. There are 18 structural isomers of octane, more including stereoisomers. Stereoisomers differ in the arrangement of atoms rather than the order of connection of atoms. For example, optical isomers have the same connections of atoms, but they are mirror images of each other – just like your two hands with the same bone structure are mirror images.
Another petroleum distillate, ISOPAR L (Ref 3), is often used in critical product cleaning. This distillation cut is designated as hydrocarbons, C11 – C 13 isoalkanes, with less than 2% aromatics. The longer the chain length, the greater the number of possible isomers. As indicated in Figure 2, dodecanes (C-12) are much larger than butane (C -4). There are 355 structural isomers of C-12 (Ref 4).
Will isoparaffins clean as effectively as your current cleaning agent? It depends. Pour paraffin hydrocarbon cleaning agent and water into a test tube or a small jar – the two liquids stay separate. Paraffin hydrocarbons, like all alkanes, are not miscible in water. Isoparaffins, like all alkanes, are miscible in oils and other non-polar compounds. Isoparaffins have a narrow solvency range; they are effective for very non-polar soils.
Some metalworking fluids and other process fluids are non-polar and are therefore soluble in isoparaffins. However, cleaning is a bit more complex; and there are many variables. Metalworking fluids change in response to heat, force, and time. The residue to be removed may be very different from the original metalworking fluid. Do you know how many synthetic and semi-synthetic metalworking fluids there are? We don’t! But there sure are a lot. The list of ingredients is often long and proprietary. Even if the original metalworking fluid is designed to be readily soluble in water, the residue after machining may be adherent and be resistant to removal by water and isoparaffins. This is why it’s important to test any new cleaning agent with your mix of products and soils.
Because isoparaffins are a mixture, the cleaning properties and possibly the materials compatibility properties can vary depending the source and on the batch. It comes down to understanding the source of the cleaning agent. The situation is analogous to purchasing a wine labelled “red blend.” Is it “Two Buck Chuck” (now actually “Three or Four Buck Chuck”)? Is it a mystery blend from a producer you’ve never heard of making claims that the wine is superb? Is it produced by a small family winery in Northern California that costs perhaps $15/bottle?
Actually sometimes, a particular batch of the mystery blend may clean very effectively. But there can be problems. Selecting a reliable supplier is crucial. We periodically find cleaning blends with an assortment of problematic ingredients. For example, the blend may contain aromatic compounds that can have safety and/or environmental issues (Ref 5). ISOPAR L is indicated to have <2% aromatics. Aromatic compounds have a very distinct structure involving planar rings and unsaturated bonds. Some aromatics, notably benzene, may clean very effectively. However, there can be health concerns. Other mystery blends may contain hydrocarbons with flashpoints that are lower than would be recommended for use in closed-loop systems.
Modified Alcohol
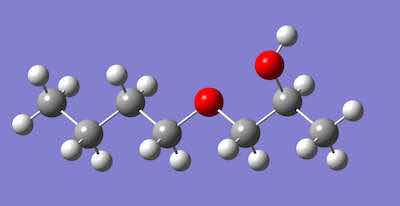 Figure 3 Modified alcohol, 1-butoxy-2-propanol, Major Component of DOWCLENE 1601.Modified alcohols are another cleaning agent being adopted by many manufacturers. Modified alcohol is not for human consumption! When Barbara hears the term “modified alcohol,” she erroneously pictures a refreshing mixed drink containing ethanol, soda, juice, fruit, and topped with a decorative paper umbrella. In the context of cleaning, modified alcohol is a molecule.
Figure 3 Modified alcohol, 1-butoxy-2-propanol, Major Component of DOWCLENE 1601.Modified alcohols are another cleaning agent being adopted by many manufacturers. Modified alcohol is not for human consumption! When Barbara hears the term “modified alcohol,” she erroneously pictures a refreshing mixed drink containing ethanol, soda, juice, fruit, and topped with a decorative paper umbrella. In the context of cleaning, modified alcohol is a molecule.
To understand modified alcohol, let’s start with water and more common, shorter chain alcohols. A water molecule contains 2 atoms of hydrogen and 1 atom of oxygen. It is designated H2O. Water is very polar; aqueous cleaning agents work to remove oils in part because they contain additives (chemicals).
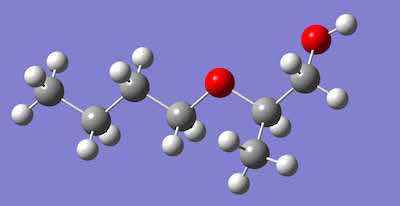 Figure 4 Modified Alcohol, 2-butoxy-1-propanol, Minor component of DOWCLENE 1601.Alcohols are organic compounds. Methyl alcohol is CH3OH; the -OH is attached to carbon by one of the four covalent bonds. Ethyl alcohol is CH3CH2OH. The general designation for an alcohol is R-OH. “R” does not designate an element; it is a general shorthand to indicate that the -OH group is bound to a carbon; The “R” indicates an unspecified carbon-containing portion of the molecule. The smaller the carbon portion of the alcohol, the greater the influence of the polar component, and the more hydrophilic (water loving) the molecule is. Larger alcohols are more like alkanes, so they are more effective at dissolving non-polar materials; the -OH polar part has less effect, making these molecules hydrophobic (water hating) like octanol, for example (CH3CH2CH2CH2CH2CH2CH2CH2OH). For many cleaning applications, even this small polar component is a useful aspect to provide effective product cleaning.
Figure 4 Modified Alcohol, 2-butoxy-1-propanol, Minor component of DOWCLENE 1601.Alcohols are organic compounds. Methyl alcohol is CH3OH; the -OH is attached to carbon by one of the four covalent bonds. Ethyl alcohol is CH3CH2OH. The general designation for an alcohol is R-OH. “R” does not designate an element; it is a general shorthand to indicate that the -OH group is bound to a carbon; The “R” indicates an unspecified carbon-containing portion of the molecule. The smaller the carbon portion of the alcohol, the greater the influence of the polar component, and the more hydrophilic (water loving) the molecule is. Larger alcohols are more like alkanes, so they are more effective at dissolving non-polar materials; the -OH polar part has less effect, making these molecules hydrophobic (water hating) like octanol, for example (CH3CH2CH2CH2CH2CH2CH2CH2OH). For many cleaning applications, even this small polar component is a useful aspect to provide effective product cleaning.
While the modified alcohol is not always identified, one SDS (Ref 6) indicates a major and minor component. Two modified alcohols are depicted in Figure 3 and 4. Oxygen is indicated in red. Please notice that there are two oxygens. One is part of an alcohol; the other is part of an ether. A general way of indicating an ether is R-O-R. At least one provider of non-linear alcohol (SAFECHEM) supplies the product with additives to inhibit acid formation. The reasoning is that chlorinated metalworking fluids could break down and become acidic.
What Happens in the Cleaning Process?
Is an isoparaffin blend or modified alcohol suitable for your critical product cleaning application? Both are marketed as replacements for chlorinated and brominated solvents. Both are structurally different from each other; and they are very different than the chlorinated solvents like trichloroethylene or n-propyl bromide (Ref 1.) They behave differently! Perhaps you may have attempted to clean with isopropyl alcohol and found it did not remove the soil residues of concern. It is important to evaluate factors like efficacy of cleaning, the potential for cleaning agent residue, and the potential for impact on materials of construction under actual process conditions.
Both are designed to be used at elevated temperature; and their behavior, including efficacy of soil removal and interaction with the substrate (the parts being cleaned) should be evaluated under expected conditions of use.
Be aware that both are used in vacuum cleaning systems (or airless/airtight systems) that are designed for combustible solvents. The cleaning systems include engineering controls that minimize employee exposure to solvent vapors and also minimize risks associated with flammable or combustible solvents. Either of these cleaning agents must be used in equipment designed for flammable or combustible solvents. This limits DIY testing. We have it on reliable authority that modified alcohol can catch fire. Fortunately, the intrepid colleague had a fire extinguisher on hand.
By Professor Darren Williams, Sam Houston State University; Barbara Kanegsberg and Ed Kanegsberg, BFK Solutions LLC
References
- “Cleaning with Organic Solvents, Part 1: Names and Structure, Ed Kanegsberg and Barb Kanegsberg” Clean Source Vol XX Issue 2
- Octane, Wikipedia https://en.wikipedia.org/wiki/Octane
- Isopar L https://echa.europa.eu/registration-dossier/-/registered-dossier/1932
- List of isomers of dodecane https://en.wikipedia.org/wiki/List_of_isomers_of_dodecane#:~:text=This%20is%20the%20list%20of%20355%20isomers%20of%20dodecane
- Aromatic compounds https://www.britannica.com/science/aromatic-compound
- SAFECHEM SDS , Dowclean 1601, Date of issue: 09/22/2017; CAS 5131-66-8 1-butoxy-2-propanol, 70-90%; CAS 15821-83-7 2-Butoxy-1-propanol <5%; http://www.efkimya.com/pdf/dowclene_msds_1601.pdf