After a recent program, a concerned attendee wanted to know: “Is there perchloroethylene in my aqueous cleaning agent?”
 Barbara and Edward Kanegsberg, Prof. Darren WilliamsBarbara’s first thought was to respond that it’s like asking “Is there chicken soup in my cupcake?” Perchloroethylene (PCE), an organic solvent, used in liquid/vapor degreasing, has a different skill set – a different set of physical and chemical properties, than do the chemicals in additive packages for aqueous cleaners.
Barbara and Edward Kanegsberg, Prof. Darren WilliamsBarbara’s first thought was to respond that it’s like asking “Is there chicken soup in my cupcake?” Perchloroethylene (PCE), an organic solvent, used in liquid/vapor degreasing, has a different skill set – a different set of physical and chemical properties, than do the chemicals in additive packages for aqueous cleaners.
It’s a great question! It’s a smart question, because a proposed federal regulation would restrict perchloroethylene usage in critical cleaning and other mixtures used in manufacturing. The answer is more nuanced than the chicken soup analogy. Important provisos: minimize exposure to all process chemicals. Cleaning agents and process chemicals are not for ingestion. Wear the appropriate protective gear like gloves and goggles.
How do you know if perchloroethylene or methylene chloride (MC), or n-propyl bromide (nPB) or trichloroethylene (TCE) is in your cleaning agent? Our clients and colleagues have immediate concerns given U.S. EPA amended TSCA activities, including proposed rules for PCE and MC that would restrict or even prohibit most uses (1,2). Allowable employee exposure, the proposed ECEL (Existing Chemical Exposure Limit, analogous to PEL or TLV), for each of the four chemicals are orders of magnitude lower than current levels – even in California. (3)
Immediate Steps
Check the label. Look at the SDS. Some SDS are more informative than others. Contact the cleaning agent supplier. Send an email, a text. Pick up the phone (gasp!); contact a person with actual technical understanding. Changes or impending changes in the additive package could impact efficacy of cleaning or materials compatibility. If there are customer requirements to never change a thing (as in many medical, aerospace, or military applications) – ask.
In fact, some brilliant cleaning agent suppliers have programs to let you know if there will be a change. SCAQMD publishes a list of Clean Air Solvents (4). Most are aqueous cleaners. Among the requirements are that the cleaning agent contain no Hazardous Air Pollutants (HAPs); perchloroethylene is a HAP.
The fact that a cleaner is certified as a Clean Air Solvent has nothing to do with how well it removes soils or how it might interact with the substrate. The cleaning agents must be tested for use in any specific application.
Vapor Degreasing and Aqueous Cleaning
Perchloroethylene is used in liquid/vapor degreasing. Some of the same properties that make it attractive for vapor degreasing make it unattractive as an additive for aqueous cleaning. In vapor degreasing, PCE is used in the washing, rinsing, and drying steps.
The washing or cleaning step is often in liquid PCE. PCE is self-rinsing; the part is held in the vapor phase, so rinsing is in freshly-distilled PCE. In contrast, aqueous cleaners contain an additive package to enhance soil removal. Aqueous cleaners are not self-rinsing; the additives that make aqueous cleaners effective are removed by rinsing in water.
In many (if not most) applications, residual water is removed by drying.
Physical and Chemical behavior
Perchloroethylene is unattractive as an aqueous additive because PCE and water don’t mix. PCE is immiscible in water; they separate into two phases. Even if PCE did mix with water, it would evaporate very differently than water. In the following table, we have shown some applicable physical properties of water, PCE, n-propyl bromide (nPB), also known as 1-bromopropane (1-BP), and isopropyl alcohol. We’ve included nPB because, while nPB is slightly soluble in water, many of the same properties hold true for both nPB and PCE.
| Property | Water | PCE | nPB (1-BP) | IPA |
| Boiling Point (Deg C) | 100 | 121 | 71 | 83 |
| Vapor Pressure 25 Deg C (kPa) | 3.2 | 1.9 | 20 | 5 |
| Heat of vaporization at boiling point (cal/g) | 540 | 51 | 59 | 160 |
| Specific Heat Capacity (cal/g °C) | 1.00 | 0.21 | 0.25 | 0.61 |
| Solubility in water (%) | 100 | 1.5 | 2.5 | 100 |
If you were putting together an aqueous cleaner, it would be unreasonable to try to use substances like PCE or nPB that are insoluble in your formulation and your rinse water. If these were to come into contact with an oily soil, such solvents would quickly leave water and move into the soil where they “feel more at home”. They would not act as a bridge to bring the soil into the aqueous cleaning solution. Only surfactants behave this way because surfactants have water-loving (hydrophilic) and oil loving (hydrophobic) portions built into their molecular structure (Figure 1).
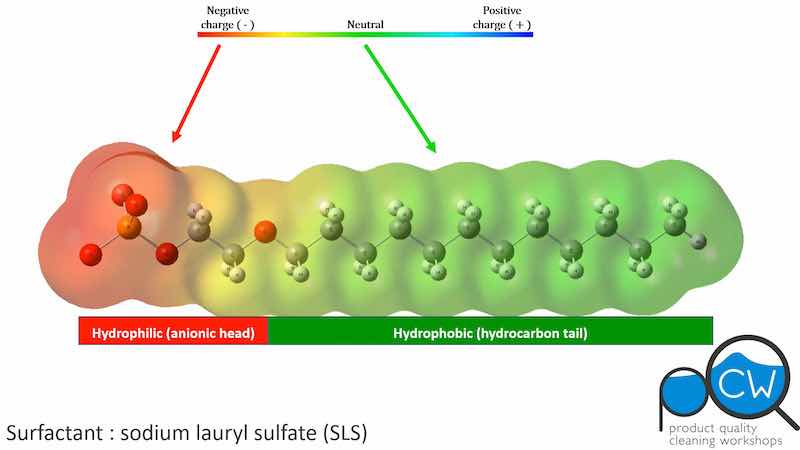 Figure 1: The structure of SLS ( CH3(CH2)11OSO3Na ) showing the hydrophilic and hydrophobic regions. (From our On-demand Aqueous Cleaning Workshop. Visit www.shsu.edu/pqcw to for more information and to register.)If you were looking for useful ingredients for an aqueous cleaning agent, those with a relatively low heat of vaporization and a low specific heat capacity would not be favored. The heat of vaporization is the energy needed to convert a liquid to a gas. Water has a heat of vaporization that is an order of magnitude higher than that of PCE or nPB. The heat capacity is the energy needed to change the temperature of a material. Water has a high specific heat capacity relative to the solvents listed; water absorbs significantly more heat to raise the temperature. PCE requires relatively low energy to raise its temperature; at the operating temperature of a process bath, PCE would volatilize. PCE would leave an aqueous mixture very rapidly. This physical property is favorable for vapor degreasing both in open-top and airless systems; in vapor degreasing, PCE is self-rinsing and residue-free drying. Lastly, any PCE or other HAPs in the process baths would have to be removed prior to release, so it would be best to never have them in the formulation to begin with.
Figure 1: The structure of SLS ( CH3(CH2)11OSO3Na ) showing the hydrophilic and hydrophobic regions. (From our On-demand Aqueous Cleaning Workshop. Visit www.shsu.edu/pqcw to for more information and to register.)If you were looking for useful ingredients for an aqueous cleaning agent, those with a relatively low heat of vaporization and a low specific heat capacity would not be favored. The heat of vaporization is the energy needed to convert a liquid to a gas. Water has a heat of vaporization that is an order of magnitude higher than that of PCE or nPB. The heat capacity is the energy needed to change the temperature of a material. Water has a high specific heat capacity relative to the solvents listed; water absorbs significantly more heat to raise the temperature. PCE requires relatively low energy to raise its temperature; at the operating temperature of a process bath, PCE would volatilize. PCE would leave an aqueous mixture very rapidly. This physical property is favorable for vapor degreasing both in open-top and airless systems; in vapor degreasing, PCE is self-rinsing and residue-free drying. Lastly, any PCE or other HAPs in the process baths would have to be removed prior to release, so it would be best to never have them in the formulation to begin with.
Isopropyl alcohol (IPA) is miscible in water; it forms a homogeneous solution with water. However, IPA is more volatile than water with a vapor pressure of 5 kPa at room temperature. IPA is not recommended as an additive to “soup up” the solvency of water because, in addition to the changing composition, there is the pesky issue of flammability (5). Even very dilute solutions of acetone and isopropyl alcohol can pose risks, especially when those solutions are heated or used with ultrasonics.
Understand – and Ask questions
Many chemicals, including organic chemicals, can be dissolved in water. Surfactants lower the surface tension of water and act as a “bridge” to allow removal of many organic (carbon-based) soils. As we have explained, formulations are complex; formulators are best viewed as artists or chefs. There could, somewhere in the universe, be an aqueous formulation, be it a solution or a stable emulsion, that contains PCE. In fact, an internet search yielded several recipes for chicken pot pie cupcakes. These might be thought of as somewhat similar to chicken soup cupcakes. However, Barbara maintains that a cupcake has a sweet cake; a cupcake has abundant frosting, and a cupcake does not include chicken or veggies. Even though an aqueous formulation containing PCE is counter-intuitive and unlikely to be offered for sale, given that EPA proposes to heavily restrict PCE, it’s best to assume nothing – ask!
References
“EPA Proposes Ban on All Consumer and Many Commercial Uses of Perchloroethylene to Protect Public Health” https://www.epa.gov/newsreleases/epa-proposes-ban-all-consumer-and-many-commercial-uses-perchloroethylene-protect
EPA Proposes Ban on All Consumer, Most Industrial and Commercial Uses of Methylene Chloride to Protect Public Health https://www.epa.gov/newsreleases/epa-proposes-ban-all-consumer-most-industrial-and-commercial-uses-methylene-chloride
“Degreasing and Fireworks,” Ed Kanegsberg and Barb Kanegsberg, in Clean Source Newsletter, July 2022. https://bfksolutions.com/degreasing-and-fireworks/
Certified Clean Air Solvents http://www.aqmd.gov/home/programs/business/business-detail?title=certified-clean-air-solvents
“Fire and Water – Acetone,” Barbara and Ed Kanegsberg in Clean Source Newsletter, May 2015 https://bfksolutions.com/fire-and-water-acetone/
Barbara and Ed Kanegsberg founded BFK Solutions in 1994 as a critical cleaning consulting service and the go-to resource to make cleaning, surface quality, and contamination problems go away, or — even better — to avoid problems in the first place. Barbara, widely known as “The Cleaning Lady,” is an expert and trusted adviser in critical cleaning. Ed is known as “The Rocket Scientist,” and they write Clean Source, an approximately monthly e-newsletter that provides practical ideas to improve cleaning, contamination control, and product quality. They are co-editors of and contributors to the acclaimed two-volume “Handbook for Critical Cleaning,” CRC/Taylor & Francis, 2011. Visit https://bfksolutions.com
Darren Williams is a Professor of Physical Chemistry at Sam Houston State University; Leader of the Cleaning Research Group; co-chair of the Product Quality Cleaning Workshops; author of articles and book chapters on cleaning verification and cleaner formulation; and performs cleaning trials and formulates cleaning chemistries at the university for external clients. williams@shsu.edu



































