The reference to “soil,” in a metal finishing context, is more than just a reference to dirt that may be present on the surface.
 Frank Altmayer MSF“Soil” encompasses any material that may be a barrier to the metallurgical bond between the base metal and the coating to be produced on top of that base metal.
Frank Altmayer MSF“Soil” encompasses any material that may be a barrier to the metallurgical bond between the base metal and the coating to be produced on top of that base metal.
A simple view of the problem can be obtained by considering all the metal atoms below the surface of the part to be processed. Each of these is atomically bonded to several other atoms nearby, creating a crystal structure. Now, picture the very last atom of metal at the surface of the part. The surface atoms do not have other like-atoms to bond to above them. Therefore, these atoms tend to bond with other available ions to obtain a stable existence. These other atoms may include oxygen (very common), sulfur (common with copper and silver), and numerous other elements.
Main image courtesy of Jayco Cleaning Technologies
Lubricants on Top of The Base Metal
On top of these “foreign” atoms bonded to the substrate to be metal-finished, manufacturing and storage lubricants are sitting on top of the base metal. Some of these are capable of forming chemical bonds with the base metal, while others merely reside on top, waiting to be removed. Of course, many facilities disassemble field-used parts (such as aircraft rework), in which case the parts may have a complex mix of soils listed, along with metal smears, oxides, and corrosion products.
While some soils are relatively easy to remove, others can be extremely difficult to remove. Easy-to-clean soils include dust, dirt, light machining oils, animal- and vegetable-based lubricants, and rust. Difficult soils include synthetic oils (especially oxidized or burned-on synthetics), buffing/polishing compounds, heat-treating scales, and any materials containing silicones (not silicates— silicones).
All of this is “soil” to the metal finisher, and the precleaning, cleaning, and acid pickling steps used before plating or anodizing are designed to remove it completely—or to the point where it no longer affects the bonding of the coating to the base metal.
Allow for Most Economical and Effective Cleaning Steps
A metal finisher should be informed about the operations performed in the various manufacturing processes used to produce the part, allowing for the most economical and effective cleaning steps. However, along with or in the absence of this information, the finisher can make his determination of soil conditions using simple tests. Is the soil oily (wet) or dry? How much particulate matter does it contain? These questions can be answered by simple wiping, touching, transferring to fingers, and rubbing.
Wet, oily soils may contain fatty acids or esters of animal, vegetable, or mineral origin. These may be distinguished by smell, especially those of organic origin when they have become rancid. Mineral oil soils are more difficult to remove. Greases are heavy-metal soaps (such as Ca, Zn, and Al stearates) and are not saponifiable or possibly not soluble in solvents, requiring alternative approaches, such as dispersion or emulsification. Dry, oily soils could contain waxes, petrolatum, or polymerized oils, which may require high-temperature cleaners to render the soil fluid.
Particulate matter (smut) dispersed in the soil may be an important consideration in the types of cleaning required. Mis-application of the cleaning cycle may make smut removal more difficult. The organic components of the soil are generally easier to remove and may leave a smut-like residue on the surface. Then electrostatic forces hold the particles more tenaciously to the surface, rendering removal more difficult.
If soil contains considerable particulate matter, therefore, it may be advisable to resort (initially) to less efficient cleaners so that particulate matter is removed along with (or in preference to) the other soil components.
Metalworking Lubricants
Parts that have been stamped, drawn, or machined will usually be smeared with lubricants. If the lubricants are known to contain vegetable oils, then agitated soaks followed by spraying might be the answer. Soils containing straight mineral oils, however, present a more difficult problem. Solvent cleaners (with or without emulsifiers), alkaline soaks, and alkaline sprays are frequently used in a proper sequence.
Parts that have been polished and buffed are considered to be difficult to clean. Materials contributing to this difficulty are abrasives, waxes, greases, fatty acids, metallic soaps, and vegetable fibers. The job of cleaning becomes even more difficult when soils have aged, as they tend to harden and dry over time. Vapor degreasing with chlorinated solvents and pressure spraying with hot alkaline solutions are commonly used methods. In a few cases, the parts are not satisfactorily clean until hand-wiped.
There are occasions when castings coated with very thin films of die lubricants are stored for considerable periods before plating. In storage, changes in humidity and temperature can cause the parts to corrode. Before polishing and buffing such parts, it is essential to remove oil and any corrosion products using an alkaline soak solution, followed by a dip in an acidic solution. Failure to do this will result in the embedding of this mix of corrosion product and oxidized lubricant into the metal's surface, creating a significant cleaning problem.
Sometimes, cleaning of parts is required before heat treatment to remove organic substances that tend to form carbonaceous material, resulting in a burnt appearance during heat treatment and subsequent quenching. Hot alkaline soak solutions are often used for this purpose. Strong acid dips are used for parts coated with heat scales. Sometimes, the steps are repeated to attain the desired results.
Frequently, protective coatings of phosphate are applied to metals to absorb lubricants (such as soaps or oils) and facilitate forming parts by drawing stamping or other fabricating operations. After the parts are formed, the remaining soils may be removed by a combination of solvents and a strong alkaline soak or chelated alkaline solutions. The latter removes the phosphate coating. Insoluble, impregnated (i.e., absorbed) soaps, however, can build up in the cleaning solution; subsequently, they are also difficult to rinse off. Filtering may effectively remove soaps and some oils, extending the useful life of the cleaner and helping to eliminate rejects resulting from inadequate stripping of the phosphate coating and cleaning.
Metalworking lubricants may be combined with other soils, such as dust, fingerprints, dirt, and corrosion products. This type of lubricant varies in composition to such a degree that it is crucial to know the type of lubricant on the part to optimize the cleaning cycle. Metalworking lubricant may contain additives, such as:
- Boundary additives
- Extreme pressure additives
- Corrosion inhibitors
- Foam suppressants
- Emulsifiers
- Dispersants
- Biocides
Boundary additives maintain lubrication when hydrodynamic lubrication breaks down. Extreme pressure additives are typically phosphorus-, sulfur-, or chlorine-based. They chemically bond to metal, minimizing friction at high-pressure loadings, and are very difficult to remove chemically.
Foam suppressants may be polyacrylate or silicone-based. Silicone-based foam suppressants wreak havoc on any metal finishing line involving electroplating by contaminating all processes and resulting in blistered deposits.
Each of the above additives may pose a unique cleaning problem. Consultation with lubricant suppliers and cleaning process suppliers is crucial in making adjustments to the cleaning cycle. The following are brief descriptions of commonly encountered lubricants:
Mineral Oils
Mineral oils are used where lubrication is of primary importance and cooling is secondary. They fall into two groups: paraffinic (straight chain) and naphthenic (ring structure). Paraffinic oils are typically used as engine oils due to their favorable viscosity index, while naphthenic oils are commonly used for metalworking because of their higher solubility for additives.
Emulsions
Emulsions are also referred to as “soluble” oils. They are typically composed of mineral oil, emulsifying agents, boundary additives, and extreme pressure additives. They are usually mixed with water, forming a “milky” white emulsion. These lubricants have good lubricity and cooling properties.
Semi-Synthetic Lubricants
Semi-synthetic lubricants are also referred to as “micro-emulsions.” They may contain small amounts of mineral oil, emulsifying agents, boundary or extreme pressure additives, foam suppressants, corrosion inhibitors, and biocides. These lubricants are more effective at cooling than reducing friction due to their high water content.
Synthetic Lubricants
Synthetic lubricants do not contain oil. They are based on polyglycol, polyisobutylene, or poly alpha-olefin compounds. These are transparent liquids containing dispersants, corrosion inhibitors, and foam suppressants. Synthetics excel at cooling over lubricating.
Natural Oils
Natural oils have either animal or vegetable origins. Vegetable oils typically consist of C12-C18 saturated and unsaturated carboxylic (fatty) acids. Animal derivative oils consist of triglycerides and fatty acids. As lubricants, fatty oils tend to be inferior to the preceding materials in all respects except boundary lubrication.
Waxes and Tallows
Waxes and tallows are common lubricating ingredients in polishing and buffing compounds. They may be of synthetic or natural origin.
Acid Pickling
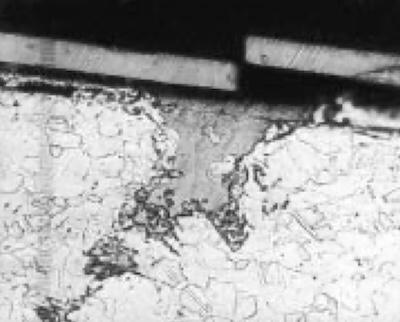 Fig. 2—Lead inclusions in brass.An acid dip is also commonly referred to as a “pickle,” although this term is typically used for the removal of heat treat scales and heavy oxides.
Fig. 2—Lead inclusions in brass.An acid dip is also commonly referred to as a “pickle,” although this term is typically used for the removal of heat treat scales and heavy oxides.
The acid dip neutralizes any alkali remaining after the rinse and solubilizes it. Light oxides, including those formed during anodic electro-cleaning, are readily removed. The acid chemically attacks the surface, providing a light or micro-etch and activation, which promotes better adhesion of subsequent deposits.
Heat treat scales or heavy oxides are removed (pickled) in either more concentrated, heated acids or in alkaline descaling solutions. Scale removal is usually done as a separate operation outside the plating line. Heat treat scales are usually mixtures of metal oxides and may require several pickling steps for removal.
Commonly encountered acid pickling problems include:
- Inappropriate choice of acid— Numerous cases of poor adhesion have been solved by substitution of the correct acid mix for the job. Lead, for example, does not dissolve well in sulfuric acid. It reacts with the sulfate to form lead sulfate, a solid material. Lead can be present in brass (see Fig. 2) and steel. Acids containing fluorides will readily dissolve the lead, generally improving adhesion. Silica films on the surface of parts can result in poor-quality deposits. Silicon is a commonly encountered alloying element in cast iron and aluminum parts. Silicon compounds are very soluble in hydrofluoric acid and fluoboric acid (HBF4).
- Galvanic displacement—More noble Metals will produce immersion deposits on substrates that are less noble. For example, steel pickled in an acid solution containing copper contamination will form an immersion deposit of copper. Nickel-plated parts can obtain an immersion deposit of gold from plating solutions that contain a high concentration of gold. These immersion deposits have a low level of adhesion when overplated. Knowledge of the galvanic series, covered earlier in this series of articles, can be used to troubleshoot suspected cases of immersion deposits.
- Inappropriate acid concentration or other operating condition— Each acid has an optimum concentration and temperature. An acid pickling problem can often be traced to a weak concentration level or operation at too low a temperature. One frequently encountered operational error is the chemical control of acid by titration with phenolphthalein, ostensibly to determine the residual or “free” acid concentration. Because this indicator changes color at a high pH (approximately 8.3), a significant amount of caustic is required for the formation of metal hydroxides, resulting in artificially high titrations and an artificially high reading of free acidity. In reality, the acid is too weak. Free acidity titrations should be made using methyl orange indicator (pH ~ 4.3), which does not pose this problem.
Frank Altmayer is a Master Surface Finisher, an AESF Fellow, and the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in the metal finishing industry. He received the AESF Past Presidents Award, the NAMF Award of Special Recognition, the AESF Leadership Award, the AESF Fellowship Award, the Chicago Branch AESF Geldzahler Service Award, and the NASF Award of Special Recognition.



































