The direct electrodeposition of coatings onto Al and its alloys is difficult due to the formation of a strongly adherent passivating oxide film.
 Authors, top row from left to right: Dr. Jing Xu, Dr. Timothy Hall, Cory Crowley, and Stephen Snyder; Second row, Dr. Brian Skinn, Dr. Maria Inman, and Dr. E. Jennings TaylorOver the past 50 years there have been numerous publications on the subject,1-7 each addressing the challenge of removing this passive oxide film such that the coating could be directly applied to the Al substrate. If this passive oxide is not properly removed, the applied coating exhibits poor adhesion, corrosion resistance, solderability or other critical performance properties.
Authors, top row from left to right: Dr. Jing Xu, Dr. Timothy Hall, Cory Crowley, and Stephen Snyder; Second row, Dr. Brian Skinn, Dr. Maria Inman, and Dr. E. Jennings TaylorOver the past 50 years there have been numerous publications on the subject,1-7 each addressing the challenge of removing this passive oxide film such that the coating could be directly applied to the Al substrate. If this passive oxide is not properly removed, the applied coating exhibits poor adhesion, corrosion resistance, solderability or other critical performance properties.
In a general sense, the most common pretreatment approaches for oxide removal involves using either zincate or stannate immersion coating processes in which the surface Al reacts with either the soluble Zn or Sn ions to deposit Zn/Sn.2 Once the Zn or Sn coatings are applied, they may be directly removed within a strip or through replacement within electroless Cu or NiP chemistries to produce a Cu or NiP deposit on the Al substrate.
A conventional Al pretreatment process is shown in Fig. 1. The processing line consists of etching, desmutting, zincating and electroless nickel phosphorus tanks that will require consistent tracking and maintenance.8-12The floor space requirements are considerable, with approximately sixteen processing tanks. Depending on the size of the parts, the tanks range in volume from 50 to 500 gallons. The rinse water tanks require regular water recovery and waste treatment cycles. Additional safety considerations are required due to the presence of HF.
Such an extensive number of processing steps and tanks requires significant capital investment for a new vendor, increases the probability of errors during the process, is time consuming (lower industrial throughput that ties up equipment) and requires large volumes of hazardous chemicals that are environmentally unfavorable and introduce cost and safety concerns. Therefore, alternative coating techniques are needed that do not require such extensive pretreatment processing with similar or better performance to conventional electroless NiP. Herein, we present a novel, single-step pretreatment process using an environmentally benign solution pretreatment approach without zincate or stannate pre-processing steps (Fig. 2). We report the material properties of Ni and/or NiP coatings directly electrodeposited onto to aluminum alloy substrates (T6061).
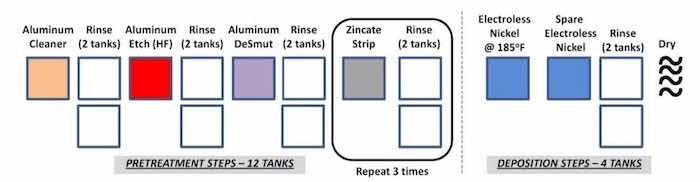 Figure 1 - Conventional electroless NiP on aluminum alloy T6061.
Figure 1 - Conventional electroless NiP on aluminum alloy T6061.
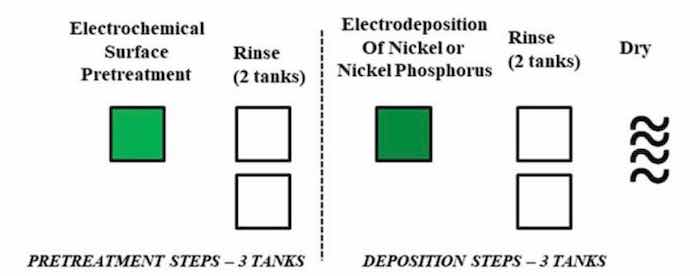 Figure 2 - Scalable five-step, environmentally-friendly electrochemical pretreatment and electrodeposition process.
Figure 2 - Scalable five-step, environmentally-friendly electrochemical pretreatment and electrodeposition process.
Experimental
Construction of electrochemical tooling for electropretreatment and deposition
Bench-scale tooling was designed and built to enable aluminum pretreatment and direct deposition of nickel (Ni; Watts Ni), or nickel-phosphorus (NiP; Umicore NIPHOS 968) onto Al alloy (T6061), as shown in Fig. 3.13-15 The complete process system (left to right) includes an electropretreatment tank (A), rinse tank (not shown) and electrodeposition tank (B) for Al (T6061) panels. The electrochemical cell incorporates thermal management (cooling coil/ heat element (not shown)), a pump for electrolyte circulation and a filter. All T6061 aluminum alloy substrates were procured from commercial sources.
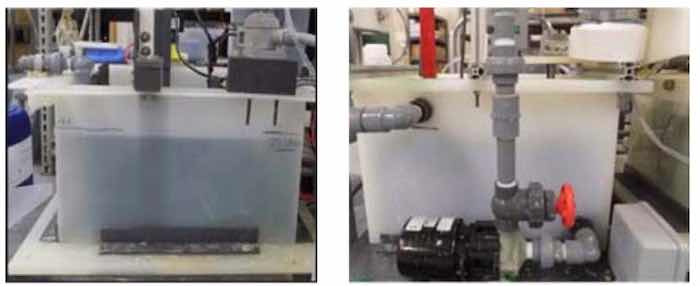 Figure 3 - Images of electropretreatment cell (A) on left above and NiP electrodeposition cell (B) on right above.
Figure 3 - Images of electropretreatment cell (A) on left above and NiP electrodeposition cell (B) on right above.
Pulse-reverse electropretreatment of 6061 aluminum
The patented electropretreatment of the aluminum alloy (T6061) surfaces (U.S. Patent Application number: 16/869,014)16 was done at a controlled temperature of 50°C using pulse-reverse voltage waveform9 in a 10% H2SO4 + 100 g/L Na2SO4 electrolyte.17 The electrochemical pretreatment process is done under pulse-reverse voltage control in order to lessen sensitivities to geometric considerations. After electrochemical pretreatment, the coupons were removed from the cell, rinsed with deionized water and transferred to the electrodeposition cell for direct electrodeposition of the targeted coatings. For all electropretreatment activities, the counter electrode used was a mixed metal oxide (MMO) coating on titanium, with an approximate anode-to-cathode gap of 3-4 inches.
Electrodeposition conditions
Throughout the course of multiple activities, we utilized various commercially available electroplating electrolytes. For direct Ni electrodeposition we prepare a conventional Watts electrolyte (pH 4.0 at 80°F) and deposited it at a current density of 4 A/dm2 using a pure Ni anode. For direct NiP electrodeposition, we procured NIPHOS 968 electrolyte (pH 2.6 at 140°F) from Umicore and electrodeposited it at a current density of 4 A/dm2 using a sulfur-activated nickel anode. After electrodeposition, the coupons were removed from the cell, rinsed with deionized water, dried by pressurized air, and photographed.
For comparison to electroplated coatings we contracted Techmetals Inc. (Dayton, OH) to apply a conventional electroless NiP with standard high phosphorus (9%-11% P) to the 6061 aluminum alloy.
Physical characterization
The coating uniformity, surface morphology and composition were investigated using a magnetic probe (Elcometer 456), XRF (Bruker, S1 TITAN Handheld) and confirmed with cross sectional analysis (Nanovea ST-400 optical (non-contact) profilometer (Nanovea, Irvine, CA)). Bend-to-break adhesion and microhardness tests were performed by Fermi National Accelerator Laboratory. MIL-A-8625F corrosion testing was done by Metallurgical Solution Inc. (MSI, Middletown, OH). Additionally, the surface finish (roughness (Ra)) before and after processing was evaluated using a contact micrometer probe (Mitutoyo SJ400 Profilometer).
Results and Discussion
Pulse-reverse electropretreatment of 6061 aluminum
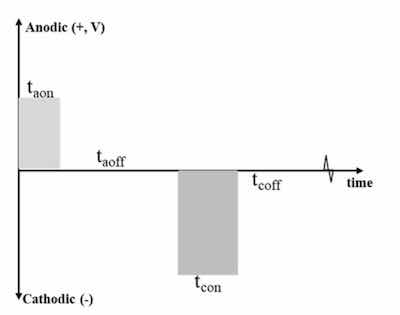 Figure 4 - Generalized pulse reverse electrochemical surface pretreatment waveform.Figure 4 shows a generalized pulse reverse electrochemical surface pretreatment waveform.18 The forward (anodic) pulse amplitude and on-time are tuned to partially oxidize the surface such that this oxide can be dissolved within the electrolyte during the reverse (cathodic) pulse amplitude, eliminating the need for hydrofluoric acid to remove the oxide.19-21 The off-time (anodic or cathodic) allows for heat dissipation.
Figure 4 - Generalized pulse reverse electrochemical surface pretreatment waveform.Figure 4 shows a generalized pulse reverse electrochemical surface pretreatment waveform.18 The forward (anodic) pulse amplitude and on-time are tuned to partially oxidize the surface such that this oxide can be dissolved within the electrolyte during the reverse (cathodic) pulse amplitude, eliminating the need for hydrofluoric acid to remove the oxide.19-21 The off-time (anodic or cathodic) allows for heat dissipation.
Utilizing our patented pulse/pulse reverse electrochemical pretreatment process, we pretreated specified 6061 T6 specimens and performed analysis on the coatings applied using standard operating conditions from the various commercially available electrolytes discussed earlier. One difference between the typical plating processes and the one used here was that, after pretreatment and rinse, a slight cathodic overpotential was applied to the sample to cathodically protect it during introduction to the plating electrolyte.
Coating visual, thickness, surface roughness and microhardness analysis
Figure 5 shows 4” × 6” 6061 Al panels coated with the commercially available deposits. Specifically, we observed that the color difference, hardness, roughness, composition and microstructure between the standard electroless NiP and the electrolytic NiP were nearly identical. When comparing these baseline materials to the nickel deposits, you can clearly see a change in the visual color and hardness, but microstructurally the coating appeared to be well adherent to the substrate.
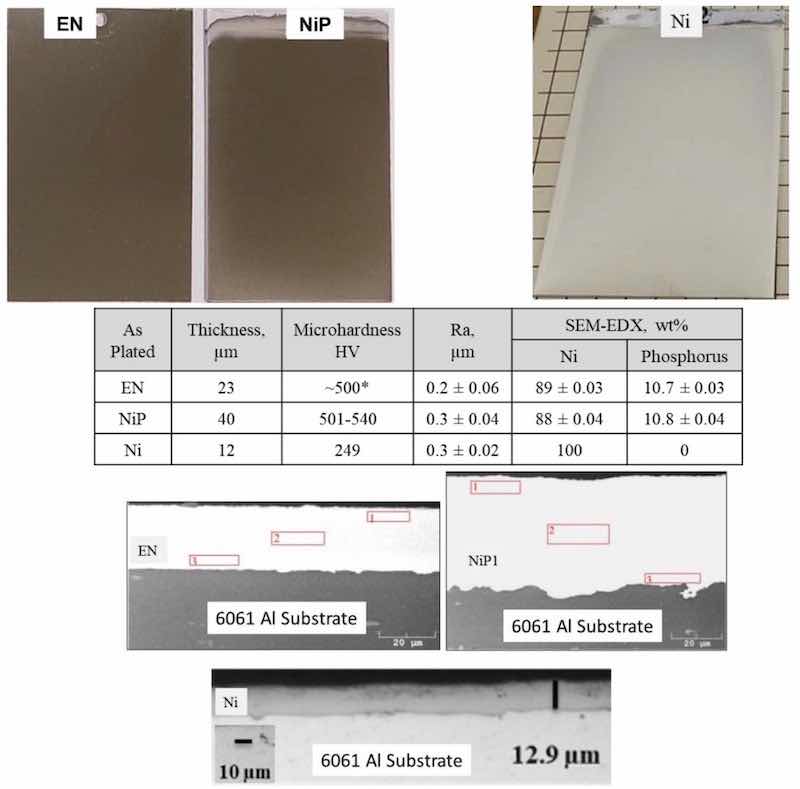 Figure 5 - (Top) Images of electroless NiP, electrolytic NiP and nickel directly applied to 4” × 6” 6061 T6 Al panels; (Middle) Table of measured deposit composition, thickness, roughness and hardness; (Bottom) Representative cross-sections for each coating system on aluminum.
Figure 5 - (Top) Images of electroless NiP, electrolytic NiP and nickel directly applied to 4” × 6” 6061 T6 Al panels; (Middle) Table of measured deposit composition, thickness, roughness and hardness; (Bottom) Representative cross-sections for each coating system on aluminum.
MIL-A-8625F corrosion test
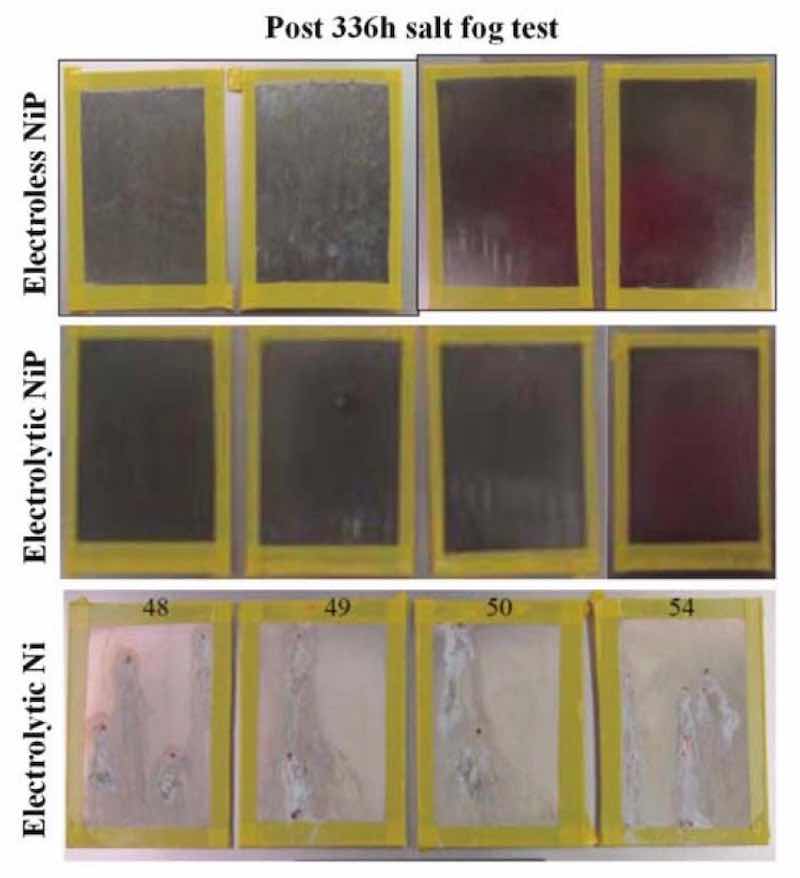 Figure 6 - Surface images of post-salt spray corrosion test electroless NiP, electrolytic NiP and electrolytic Ni applied to 6061 Al.Salt spray testing was performed in accordance with MIL-A-8625F by an accredited facility MSI (Middletown, OH; accredited A2LA). The detailed procedures used by MSI to perform the test were: (1) clean panel surfaces with acetone; (2) install panels in cabinet at ~6o from the vertical and parallel to the flow of the fog, in accordance with MIL-A-8625F paragraph 4.5.3.1 (with an exposed area of 15 in2); (3) run the test on all panels to termination (which is 336 hr per MIL-A-8625F); (4) after testing, rinse the panels with running distilled water at less than 100oF; (5) examine the surfaces with an Olympus SZ61 stereoscope with Pax-it image analysis software to look for corrosion and blistering and (6) count the number of pits per ASTM E29 for the NiP and Ni samples.
Figure 6 - Surface images of post-salt spray corrosion test electroless NiP, electrolytic NiP and electrolytic Ni applied to 6061 Al.Salt spray testing was performed in accordance with MIL-A-8625F by an accredited facility MSI (Middletown, OH; accredited A2LA). The detailed procedures used by MSI to perform the test were: (1) clean panel surfaces with acetone; (2) install panels in cabinet at ~6o from the vertical and parallel to the flow of the fog, in accordance with MIL-A-8625F paragraph 4.5.3.1 (with an exposed area of 15 in2); (3) run the test on all panels to termination (which is 336 hr per MIL-A-8625F); (4) after testing, rinse the panels with running distilled water at less than 100oF; (5) examine the surfaces with an Olympus SZ61 stereoscope with Pax-it image analysis software to look for corrosion and blistering and (6) count the number of pits per ASTM E29 for the NiP and Ni samples.
The coated 6061 panels after the specified salt spray corrosion testing are shown in Fig. 6, while Table 1 shows the results of the post corrosion analysis. The data indicate that both the electroless NiP and DC electrodeposited nickel phosphorus-coated samples passed the corrosion test (MIL-A-8625F paragraph 6.19 and 3.7.1.2) identified over 150 in2. However, the nickel-coated samples did not pass the pitting requirements for the test.
| Coupon Type | Coupon Label | Number of Pits | Corrosion Test Time (h) |
| Electroless NiP | EN7 | 0 | 336 |
| Electroless NiP | EN8 | 0 | 336 |
| Electroless NiP | EN9 | 0 | 336 |
| Electroless NiP | EN10 | 0 | 336 |
| Electrolytic NiP | 106 | 0 | 336 |
| Electrolytic NiP | 108 | 0 | 336 |
| Electrolytic NiP | 109 | 0 | 336 |
| Electrolytic NiP | 110 | 0 | 336 |
| Electrolytic Ni | 81 | 6 | 336 |
| Electrolytic Ni | 83 | 3 | 336 |
| Electrolytic Ni | 84 | 0 | 336 |
| Electrolytic Ni | 85 | 5 | 336 |
Bend test
 Figure 7 - From left to right is 1) the device for the Bend test; 2) the inflection point of the electroless NiP (EN1, EN2); 3) electrodeposited nickel (16, 17) and electrodeposited nickel phosphorus (96, 102) coupons after the 0.5” bend radius test.The Bend test was performed at Fermilab, by Mr. Cory Crowley. The initial bend tests were done with a 0.5” bend radius on the flat panels, as shown in Fig. 7. Both the electroless nickel phosphorus (EN1, EN2), the electrolytic nickel (16, 17) and the electrolytic nickel phosphorus (96, 102) passed the test and showed good adhesion as depicted in Fig. 7 and Table 2.
Figure 7 - From left to right is 1) the device for the Bend test; 2) the inflection point of the electroless NiP (EN1, EN2); 3) electrodeposited nickel (16, 17) and electrodeposited nickel phosphorus (96, 102) coupons after the 0.5” bend radius test.The Bend test was performed at Fermilab, by Mr. Cory Crowley. The initial bend tests were done with a 0.5” bend radius on the flat panels, as shown in Fig. 7. Both the electroless nickel phosphorus (EN1, EN2), the electrolytic nickel (16, 17) and the electrolytic nickel phosphorus (96, 102) passed the test and showed good adhesion as depicted in Fig. 7 and Table 2.
Bend tests were also performed using a 0.25” bend radius on 1” × 4” × 0.1” 6061 Al panels, with the primary goal of achieving adhesion failure of the coating. Both the electroless NiP coupons and electrolytic Ni passed the test, though electrolytic NiP showed adhesion failure on the edges of the coupons, as shown in Fig. 8 and summarized in Table 3. The surface cracking on the edges of the flat electrolytic nickel phosphorus panels is a phenomenon that typically is eliminated when plating actual shaped components, e.g., cylindrical shapes, because most parts are without sharp edges, or high current density regimes.
| Coupon | Process | Observation |
| EN1 | Electroless Nickel from Techmetal | Pass |
| EN2 | Electroless Nickel from Techmetal | Pass |
| 16 | Ni electrodeposition after 1-step electropretreatment | Pass |
| 17 | Ni electrodeposition after 1-step electropretreatment | Pass |
| 96 | Ni electrodeposition after 1-step electropretreatment | Pass |
| 102 | Ni electrodeposition after 1-step electropretreatment | Pass |
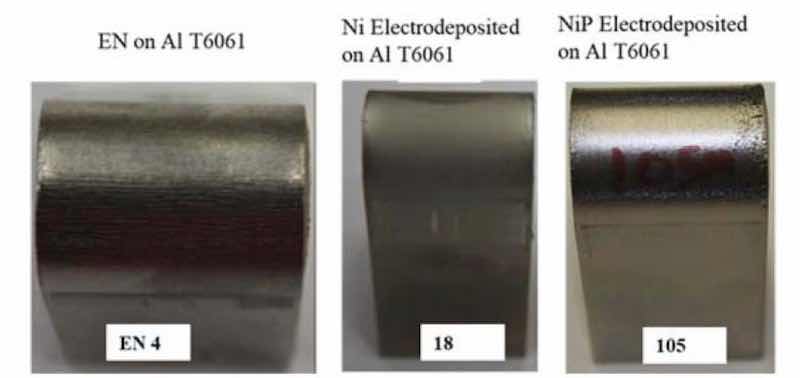 Figure 8 - Left to right: The inflection point of the electroless NiP (EN4), electrolytic Ni (18) and electrolytic NiP (105) coupons after the 0.25” bend radius test.
Figure 8 - Left to right: The inflection point of the electroless NiP (EN4), electrolytic Ni (18) and electrolytic NiP (105) coupons after the 0.25” bend radius test.
| Coupon | Process | Observation |
| EN4 | Electroless Nickel from Techmetal | Pass |
| 18 | Ni electrodeposition after 1-step electropretreatment | Pass |
| 105 | Ni electrodeposition after 1-step electropretreatment | Coupon edge blister |
Wear test
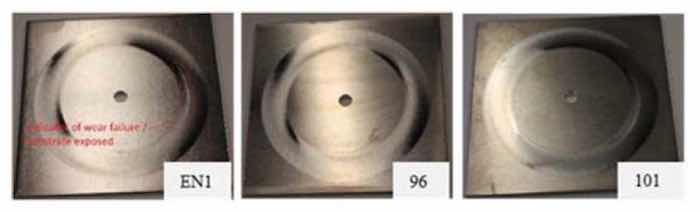 Figure 9 - Images of electroless NiP (EN 1) and representative electrolytic NiP (96, 101) coupons after the taber wear test, undertaken at FermiLabThe Taber wear testing was performed at Fermilab using a 5135 Abraser per ASTM D4060. The wear index trials test conditions are as described in Table 4 (Left). The measured Taber wear index number (mass loss/# cycles /1000)) measured until coating breakthrough is shown in Table 4, Right. The exemplar coating breakthrough for representative sample tested is shown in Fig. 9, where the aluminum layer that had been broken through has a noticeably whiter appearance as compared to the nickel phosphorus coating. The measured Taber wear index number at runout for each sample tested is very similar except for coupon 102. Overall, since wear is related to the hardness and microstructure of the material in contact with the wheel, this wear index number similarity between ENi and NiP electrodeposited coupons indicates their materialistic similarities.
Figure 9 - Images of electroless NiP (EN 1) and representative electrolytic NiP (96, 101) coupons after the taber wear test, undertaken at FermiLabThe Taber wear testing was performed at Fermilab using a 5135 Abraser per ASTM D4060. The wear index trials test conditions are as described in Table 4 (Left). The measured Taber wear index number (mass loss/# cycles /1000)) measured until coating breakthrough is shown in Table 4, Right. The exemplar coating breakthrough for representative sample tested is shown in Fig. 9, where the aluminum layer that had been broken through has a noticeably whiter appearance as compared to the nickel phosphorus coating. The measured Taber wear index number at runout for each sample tested is very similar except for coupon 102. Overall, since wear is related to the hardness and microstructure of the material in contact with the wheel, this wear index number similarity between ENi and NiP electrodeposited coupons indicates their materialistic similarities.
| Test Date | 06/11/2019 |
| Temperature F | 70.7F |
| Humidity (%RH) | 63% |
| Abrading Counterweight | 750 g |
| Speed Control | 60 RPM |
| Vacuum Power | 100% |
| Abrading Disc Type | CS-10 |
| Recondition Frequency | 500 Cycles |
| Coupon | Process | Wear Index @ Failure Cycles mg/1000 cycles |
| EN1 | Electroless Nickel | 23.6 |
| 96 | NiP Electrodeposited on Al T6061 | 22.7 |
| 101 | NiP Electrodeposited on Al T6061 | 23.2 |
Conclusion
In summary, we have successfully demonstrated the potential of a scalable five-step environmentally-friendly electrochemical pretreatment process that can be utilized to prepare 6061 aluminum alloy for direct electrolytic deposition of nickel and NiP without the need for zincate or stannate pretreat approaches. Reports herein demonstrate similar metallurgical, corrosion and material properties for electrolytic NiP and electrolytic Ni directly applied to Al when compared to electroless NiP coatings prepared through a zincate process. Therefore, this new approach to prepare Al for direct deposition can significantly reduce the number of processing steps, hazardous chemistry use, cost and capital investment. Furthermore, this electrolytic deposition process is much more rapid and less sensitive to chemical changes, enabling better part throughput. Currently, we are continuing to optimize the electropretreatment and electrodeposition process to further improve the coating properties and enable process scalability.
Acknowledgements
This material is based upon work supported by the Department of Energy under Grant No. DE-SC0017751 and US Air Force under Grant No. FA8501-16-P-00479. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Department of Energy.
About the Authors
Dr. Jing Xu is currently a Principal Engineer at Global Foundries. She received her B.S. from Ludong University, Master’s degree from Nanjing University and her Ph.D. from Case Western Reserve University in Chemistry (spectroelectrochemistry) in 2015.
Dr. Timothy D. Hall is the Laboratory Manager at Faraday Technology Inc. He received his B.S. in Chemical Engineering and Mathematics from West Virginia University (Morgantown, WV) in 2003, his M.S. and Ph.D. in Chemical Engineering from the University of Notre Dame (Notre Dame, IN) in 2006 and 2007, respectively. Dr. Hall was part of a team that received a 2011 R&D 100 Award, 2013 green chemistry award, and was a 2016 R&D 100 Award finalist in both plating and surface finishing electrochemical technologies. He has been a significant contributor to work that has led to six patents and numerous pending patent applications.
Cory Crowley is a Senior Mechanical Engineer at Fermi National Accelerator Laboratory. He received his B.S. in Mechanical Engineering from Northern Illinois University (DeKalb, IL) in 2008, and has served in several FNAL divisions for 10+ years. Cory currently serves as an engineering lead and technical manager on focusing components for the Long Baseline Neutrino Facility.
Stephen T. Snyder is a Senior Research Engineer at Faraday Technology, Inc. He received his B.E. degree in Chemical Engineering from the University of Dayton and his M.S. degree from Purdue University in Materials Engineering. Mr. Snyder implements the engineering, design and fabrication of prototype instruments at the Faraday facility.
Dr. Brian Skinn is a Principal Research Scientist at Faraday Technology, Inc. He received his B.S. degree in Chemical Engineering from Case Western Reserve University in 2004, and studied transport phenomena and reaction kinetics with William Deen at MIT, graduating with a Ph.D. Chemical Engineering in December 2011. He has played a lead role in development of methods for zero-discharge recycling of electrochemical machining effluent and leads Faraday’s efforts in Multiphysics simulation of electrochemical processes. In addition, he played key roles in activities focused on dewatering of algae for biofuel production, lignin oxidation for production of high value added products, CO2 reduction to value-added products, electrodeposition of functionally-graded brazing interlayers for nuclear fusion first-wall components, and electrochemical destruction of poly-/perfluorinated species (PFAS) in industrial/landfill effluents and soil matrices for environmental applications.
Dr. Maria Inman is the Research Director at Faraday. Dr. Inman leads Faraday’s research and development function. In addition to providing day to day direction to the science and engineering staff at Faraday, Dr. Inman has served as Principal Investigator on millions of dollars worth of government and commercially-funded projects and serves as an integral member of Faraday’s internal strategic planning and IP management group. Dr. Inman has been on staff at Faraday for 26 of its 30 years in business.
Dr. E. Jennings Taylor uniquely blends 40+ years entrepreneurial business experience with demonstrated skills in technology innovation and intellectual asset analysis. Prior to forming Faraday, Dr. Taylor held positions at Giner, Inc. as the Manager of Fuel Cell Research (1982-1985), and at Physical Sciences where he held numerous positions including the Manager of Electrochemical Technologies (1985-1991). In 1991, EJ left Boston to form Faraday Technology, Inc. He successfully secured start-up funding and from 1991-1997 served as the Principal Investigator on many of Faraday’s early research projects. In 1997, Dr. Taylor shifted his emphasis from research to strategic corporate direction and technology portfolio management. In order to facilitate the development of an intellectual property portfolio, he studied to become a Patent Agent and in February, 2003 was granted the status of registered agent with the US Patent and Trademark Office. Dr. Taylor applies this skill to develop patent portfolios that can benefit potential customers. EJ is well recognized in both the professional and business community. He is co-chair of the Technical Advisory Committee for SURFIN/NASF, Chair of the NASF/AESF Foundation Reserch Board, a past Treasurer of the Electrochemical Society, and a past Chair of the SBIR Advisory Board of the National Science Foundation.
References
- G. Sheasby and R. Pinner, The Surface Treatment and Finishing of Aluminum and its Alloys, 6th Edition, ASM International, Materials Park, Ohio; Finishing Publications Ltd., Teddington, Middlesex, UK, 2001.
- S. Lashmore, “Plating on Aluminum: A Review,” Plating & Surface Finishing, 72 (6), 36-39 (1985).
- Loch, “Tank Process for Plating Aluminum Substrates Including Porous Aluminum Castings”, US Patent 4,346,128, Aug 1982.
- W. Bibber, “Zincate or Stannate-free Plating of Mg, Al and Ti,” Metal Finishing, 107 (7-8), 28-30 (2009).
- Pearson and S.J. Wake, “Improved Pretreatment of Aluminium as a Substrate for Electrodeposition” Aluminium Today, 1997; also Trans. IMF, 75 (3), 93-97 (1997).
- E. Such and A.E. Wyszynski, “An Improvement in the Zincate Method for Plating on Al”, Plating, 52 (10), 1027-1034 (1965).
- W. Golby and J.K. Dennis, “A Study of the Effect of Pretreatment Procedures on the Plating of Aluminum Alloys,” Surface Technology, 12 (2), 141-155 (1981).
- Composition for Desmutting Aluminum, US Patent US6407047 B1, 2002.
- Saleema, et al., “A simple surface treatment and characterization of AA 6061 aluminum alloy surface for adhesive bonding applications,” Applied Surface Science, 261, 742-748 (2012).
- Honma, H. Watanabe and T. Kobayashi, “Direct Nickel Plating on Aluminum Substrate for Microbump Formation”, J. Electrochem. Soc., 141 (7), 1791-1794 (1994).
- J. Monteiro, et al., “Surface Pretreatments of Aluminum for Electroplating”, Surface and Coatings Technology, 35 (3-4), 321-331 (1988).
- G. Gonzalez Gutierrez, M.A. Pech-Canul and P.J. Sebastian, “Zincating Effect on Corrosion Resistance of Electroless Ni-P Coating on Aluminum Alloy 6061,” Fuel Cells, 17 (6), 770-777 (2017).
- Xu, et al., “Direct Electrodeposition Onto 6061 Aluminum by One Step Surface Pretreatment”, Oral Presentation at the 2019 NASF SUR/FIN Manufacturing and Technology Tradeshow and Conference, Chicago, IL, June 2019.
- Xu, et al., “Direct Electrodeposition onto 6061 Al by one step surface pretreatment”, Oral Presentation at the 2018 NASF SUR/FIN Manufacturing and Technology Tradeshow and Conference, Cleveland, OH, June 2018.
- Xu, et al., “Direct Electrodeposition on 6061 Aluminum by One Step Surface Pretreatment” ASM International MS&T 2018, Columbus, OH
- D. Hall, et al., “Electrolytic Preparation of a Metal Substrate for Subsequent Electrodeposition” US Pat. Appl. No. 16/869,014 filed May 7, 2020.
- Xu, et al., “Pulse Reverse Plating of Zn-Ni on Aluminum and Steel.” ECS Transactions, 77 (11) 1237-1245 (2017).
- J. Taylor “Adventures in Pulse/Pulse Reverse Electrolytic Processes: Explorations and Applications in Surface Finishing” J. Applied Surface Finishing, 3 (4), 78-189 (2008).
- J. Taylor and M.E. Inman, “Electrodeposition of Catalytic Metals Using Pulsed Electric Fields,” US Patent 6,080,504, 2000.
- J. Taylor, M.E. Inman and T.D. Hall, “Electrochemical system and method for electropolishing hollow metal bodies,” US Patent No. 9,987,699, 2018
- J. Taylor, “Sequential electromachining and electropolishing of metals and the like using modulated electric fields,” US Patent 6,558,231, 2003.



































