I will provide you with the basics of cyanide safety, but I must caution anyone reading this and any following articles on the topic that cyanide safety is discussed here in a general manner.
 Frank AltmayerYour specific process may have safety issues that will not be covered here, so don’t count on this as a “complete” discussion of the issues. Also, I am not a doctor, so any information given here is not an attempt at practicing medicine. Check with your own doctor for a specific diagnosis.
Frank AltmayerYour specific process may have safety issues that will not be covered here, so don’t count on this as a “complete” discussion of the issues. Also, I am not a doctor, so any information given here is not an attempt at practicing medicine. Check with your own doctor for a specific diagnosis.
First, let me state that cyanide has been used in metal finishing for over 150 years, and the number of casualties related to accidental poisoning with cyanide in an industrial setting is relatively low.
During my career, I have only heard/read of a handful of workers dying from cyanide poisoning. In many of these cases, the poisoning was self-inflicted (suicide) or the result of extreme carelessness. For example, several workers were killed when one of them added concentrated acid to a cyanide-containing crust on an empty tank during clean-up.
Cyanide can be and is being used in the metal finishing industry on a daily basis, with a high level of safety.
Cyanide Basics
Cyanide is an anion that is a combination of one carbon and one nitrogen atom (CN). Carbon and nitrogen are relatively harmless chemical elements, but when they are combined in this atomic ratio, a potentially deadly anion is produced. Cyanide-ion-containing compounds are commercially produced, and more than 90% of worldwide production is used in the mining industry. Only a very small percentage is used in metal finishing.
Nature also produces cyanide, as some bacteria, fungi, and algae produce cyanide as a by-product as they live and die. Certain plants such as peach pits, almonds, spinach, tapioca, and bluegrass contain naturally produced (very low concentrations) cyanide compounds.
Cyanide-containing compounds fall into two broad categories: simple and complex.
Simple Cyanides
In general, simple cyanides are considered to be highly toxic and relatively unstable, especially when acidified or combined with oxidizers, while complex cyanides have higher levels of stability and may have lower levels of toxicity, depending on the composition.
Simple cyanide examples are potassium and sodium cyanide (KCN and NaCN). These are the most commonly encountered cyanide compounds in metal finishing. Sodium and potassium cyanide are white crystalline compounds that may also be compressed into more easy-to-handle shapes such as “eggs” or “briquettes.” These compounds give off a bitter almond “nutty” smell, but not everyone has the ability to detect this odor.
Sodium and potassium cyanide are easily dissolved in water and are highly toxic via ingestion. As little as 6.4 mg/kg of body weight kills 50% of a rat population in experimental investigations. That is equivalent to a 220-pound man ingesting only 0.64 grams or 0.02 ounces.
Hydrogen cyanide may also be considered a simple cyanide. It is a gas that is produced by accidentally mixing acid or an oxidizer and simple or complex cyanide. Hydrogen cyanide is not intentionally used in metal finishing. It is a colorless gas that also has a weak, bitter almond smell, but again, not everyone can detect this odor.
Complex Cyanides
In general, complex cyanides tend to be stable, and many are not soluble in water. Complex cyanide compounds tend to resist decomposition upon the addition of acid and do not readily produce hydrogen cyanide unless the acid added is strong, or the complex compound is heated to high temperatures. Some complex cyanides are so stable and low in toxicity that they have been used to color blue jeans and as anticaking agents for road salt.
Examples of complex cyanides are potassium ferricyanide (K3Fe[CN]6) and potassium gold cyanide (KAu[CN]2). There are many dozens of complex cyanide-containing chemical compounds, but only a few are utilized in the metal finishing industry.
Uses of Cyanide in Metal Finishing
In metal finishing, the most common processes that employ cyanide ions are electroplating and stripping operations, some of which are listed in Table 1. In electroplating solutions, sodium or potassium cyanide may be included in the process formulation to allow alloy deposition, improve solution conductivity, control metallic impurities, and dissolve metallic anodes.
In stripping solutions, sodium or potassium cyanide is utilized to chemically combine with the metal being stripped, resulting in the dissolution of the plated metallic layers. By the incorporation of suitable inhibiting agents, these stripping solutions are fast, operate at room temperature, and maintain their speed of stripping for a long time. It should be noted, however, that all stripping solutions are not necessarily formulated with cyanide.
Health Issues
Exposure Routes
Cyanide poisoning may result from exposure via several routes, but inhalation and ingestion are the most common ones.
Inhalation
There are two physical forms of cyanide-bearing air emissions that are possible from metal finishing processes containing cyanide: particulate and gas. Particulates containing cyanide are typically mists that may be produced by gases or air bubbles bursting at the surface of a process solution. Under most circumstances, these emissions are captured and removed from the air stream by exhaust and scrubbing systems.
Most metal finishing processes containing simple cyanides are stabilized by the addition of large amounts of alkalis, such as sodium or potassium hydroxide, so there is little, if any, possibility of gaseous hydrogen cyanide being formed due to decomposition.
NIOSH studied particulate emissions on several cyanide-containing electroplating solutions in 1984 (NIOSH publication 85-102) and found that in general, personal exposure to particulate cyanide emissions was below detection in ventilated and unventilated electroplating tanks.
The main inhalation danger is from accidental mixing of any acidic solution and a cyanide-containing solution, yielding gaseous hydrogen cyanide. Industrial fires in metal finishing shops can also release cyanide gases. Inhalation of smoke from such a fire can result in cyanide exposure.
Workers who smoke cigarettes routinely expose themselves to long-term exposures to low concentrations of cyanide (note the Surgeon General’s warning on packs of cigarettes). Such long-term exposures may increase the vulnerability of smokers to cyanide toxicity and can cause some of the symptoms of over-exposure noted below.
Ingestion
Ingestion of cyanide-containing compounds is typically a result of carelessness on the part of the worker. Failure to utilize appropriate personal protective equipment (PPE) or failure to wash hands before eating or smoking can result in the ingestion of cyanide-containing materials.
Skin/Eye Contact
Cyanide may be absorbed through the skin or membranes of the eye. Therefore, the use of protective gloves, goggles (face shield if splashing or dust exposure is a possibility), boots, eye protection, and impervious clothing is very important when working with these compounds and solutions.
Health Effects
The primary mechanism by which cyanide impairs the proper function of internal organs is the combination of cyanide with the hemoglobin in the bloodstream. This prevents hemoglobin from properly oxygenating bodily cells, effectively starving the organs of oxygen. Cyanide-bearing solutions are also highly caustic, producing chemical burns upon skin contact, inhalation, or ingestion.
The route of exposure to cyanide does not change the health effects/symptoms of cyanide poisoning to any significant degree, with the exception of skin contact. Exposure through skin contact may result in sores and rashes of the skin. Symptoms similar to inhalation and ingestion may be slower to show when the route is skin contact.
Signs of low-level exposure to cyanide include the skin turning pink to red. High levels of exposure may turn the skin blue. Reddening of the eyes and pupil dilation may be evident.
Exposure to high concentrations of airborne or ingested cyanide can impair the proper function of numerous organs, including the liver, kidneys, heart, and central nervous system, and can cause coma and death.
OSHA regulates worker exposure to inhaled cyanide at 5 mg/m3 of air on an 8-hour time-weighted average.
Ingestion of cyanide typically results in rapid deep breathing/shortness of breath. If the ingestion is above the toxic amount, convulsions, loss of consciousness, and death can result in a matter of minutes.
Exposure to cyanide at levels that are not toxic may still cause serious health effects, including breathing problems, chest pain, headaches, damage to the thyroid gland, and gastric irritation.
To the best of my knowledge, long-term exposure to cyanide has not been linked to cancer or birth defects.
Antidote for Cyanide Poisoning
OSHA requires an employer to have on-hand first aid for any foreseeable injury. There is a first aid “kit” available that includes the chemicals and equipment needed for first aid response. The kit has detailed instructions for first aid response. Find more info HERE.
Except for the pearls of amyl nitrite, the kit contents are designed and intended for use by a medical professional. However, since time is of the essence in a cyanide incident, workers should be trained on how to utilize the amyl nitrite pearls in an emergency. To avoid abuse of amyl nitrite, it should be stored in a “break glass” box. The pearls should not be locked up, as that
Cyanide and Heat
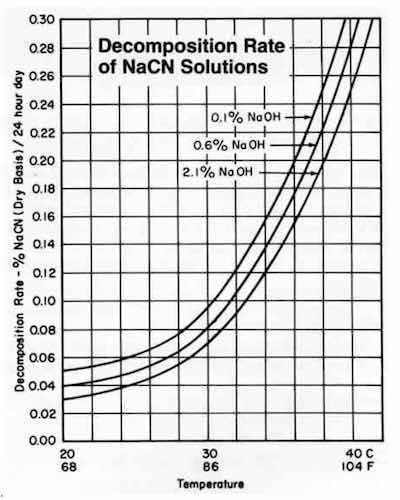 Figure 1Solid sodium cyanide decomposes very rapidly when heated above about 140°F (60°C) (See Fig. 1 from DuPont). The decomposition products include cyanide gas, ammonia, and oxides of nitrogen. Storage in rooms that are conditioned to remain below a safe 120°F (49°C) or lower should be utilized. Do not store in a room where the temperature may exceed the critical temperature at any time.
Figure 1Solid sodium cyanide decomposes very rapidly when heated above about 140°F (60°C) (See Fig. 1 from DuPont). The decomposition products include cyanide gas, ammonia, and oxides of nitrogen. Storage in rooms that are conditioned to remain below a safe 120°F (49°C) or lower should be utilized. Do not store in a room where the temperature may exceed the critical temperature at any time.
Once the cyanide is mixed with water, the solution may be heated above 140°F (60°C), but the higher it is heated, the more it tends to decompose, usually producing ammonia and carbonates.
When using electric immersion heaters to heat a process containing cyanide, failure to maintain proper volume level can expose the tank and the solution to excessive heat, resulting in decomposition of the cyanide and a possible combustion hazard, particularly when plastic tanks are used. For this reason, it is not a good idea to use electric heaters on cyanide-bearing process tanks, even with over-temperature sensors.
Is Cyanide Explosive?
Cyanide is not considered an explosion hazard, but like many chemicals, if mixed with the “wrong” stuff, an explosion can result. First, a sealed container of cyanide can “explode” if heated to the decomposition temperature. This is very important for
firefighters. In a fire, containers of cyanide need to be cooled. This is more complicated than it sounds. Water can be used to cool the containers, but water should not be used to fight fires if cyanide containers are ruptured (use foam instead). Foam has no cooling capacity, so it should not be used to try to cool closed containers. Cyanide is also incompatible with carbon dioxide extinguishers because carbon dioxide and moisture produce carbonic acid, which can produce cyanide gas.
In a fire, cyanide can explode if mixed with oxidizers such as chlorates or nitrites, so these need to be stored as far apart as possible. Other incompatibles with cyanide include all acids, nitrates, fluorine, magnesium, and all oxidizers.
Cyanide and Moisture
 Figure 2: Workers must be trained to replace the metal ring and tighten it with a wrench.You will notice that cyanide is typically delivered in a drum with a steel ring that keeps a tight seal on the product (Fig. 2). That is because sodium and potassium cyanide are hygroscopic. That means they draw moisture from the air if the container is left open. Workers must be trained to replace the metal ring and tighten it with a wrench. Do not just put the metal lid on the container.
Figure 2: Workers must be trained to replace the metal ring and tighten it with a wrench.You will notice that cyanide is typically delivered in a drum with a steel ring that keeps a tight seal on the product (Fig. 2). That is because sodium and potassium cyanide are hygroscopic. That means they draw moisture from the air if the container is left open. Workers must be trained to replace the metal ring and tighten it with a wrench. Do not just put the metal lid on the container.
Cyanide reacts with moisture to produce cyanide gas, ammonia, and formate. It should not be stored underneath a sprinkler system. The storage area should be equipped with ventilation and a cyanide gas detector, if possible.
Cyanide and Empty Containers
Empty containers of cyanide must be triple-rinsed before the container is considered to be truly empty by the USEPA.
Cyanide and Acid-Bearing Processes
It is not unusual that a cyanide-containing metal finishing process has an acid-containing process nearby. This poses several safety issues that need to be addressed:
- OSHA requires that cyanide and acid that may mix with cyanide be diked off to prevent an accidental simultaneous spill from both tanks from combining to produce toxic gas. This is often a curb or collection tray located underneath one of the tanks (and the rinse tanks as well). Such trays do not prevent a pinhole leak in the side of the tank from “spraying” outside the containment. In some such cases, double-wall tanks have been employed.
- Management needs to consider the possibility of a worker accidentally making an acid or cyanide addition to the wrong tank. Color coding, locked lids, or additions made by two people (one adds while the second confirms the correct tank) are some of the management practices that have been utilized in some facilities.
- The rinses after acid must not be allowed to mix with the rinses after the cyanide process. Designated, color-coded plumbing should be employed, and the rinses after cyanide need to be properly routed to a wastewater treatment system designed to destroy cyanide residuals.
Cyanide and Worker Health Issues
 Figure 3We discussed the high toxicity of cyanide and mentioned the primary routes of entry and health effects of cyanide exposure. This month, we expand on the subject some more by suggesting that workers be given a medical exam prior to their employment near and with cyanide.
Figure 3We discussed the high toxicity of cyanide and mentioned the primary routes of entry and health effects of cyanide exposure. This month, we expand on the subject some more by suggesting that workers be given a medical exam prior to their employment near and with cyanide.
Workers with pre-existing conditions such as central nerve damage, thyroid conditions, or skin, heart, or lung diseases should not be employed where cyanide exposure is a possibility.
We also mentioned that as little as 0.64 grams of sodium cyanide can cause death by ingestion in a 220-pound person. Figure 3 emphasizes just how little this amount is. P&SF
In Case of a Spill
Spills need to be evaluated for size. A spill of a few gallons that is isolated and has not had a chance to mix with anything else presents a limited risk of overexposure and may be cleaned up by trained personnel upon notification. This may involve the use of a bucket and mop that is used only for such spills and is cleaned after each and every use (including a final soak in a dilute solution of water and sodium hypochlorite in a ventilated area). You may prefer to use solid absorbents such as sand or proprietary materials. In any case, the spill/absorbent needs to be disposed of as hazardous waste or treated for cyanide destruction.
In most facilities, it is the Emergency Coordinator’s job to decide if a spill meets this criterion.
A spill that is large enough or any other spill that has had a chance to mix with incompatible chemicals calls for an evacuation. It is also a wise choice to evacuate any time there is any doubt as to whether it is safe to stay around a spill. Obviously, if anyone smells cyanide gas, it’s also time for an evacuation.
Employees should be instructed to:
- Not allow a spill to run to a drain that goes to the sewer
- Not spray a spill with a high-pressure hose
- Not allow a spill to enter a confined space, if possible
Any spill that involves the cyanide leaving the facility property boundaries needs to be evaluated to determine if a report to the National Response Center is mandated (one pound or more of cyanide in the spill is the cut-off).
Evacuation
Employees need to be trained to evacuate using the nearest exit. They must gather at a pre-designated spot that is safely far enough away from the facility. If possible, this pre-designated spot should be upwind of the prevailing direction that the wind takes at the facility. It is extremely important that employees are trained to remain at the designated gathering spot because if employees wander off-site, it is impossible to verify that all are outside and none are trapped or injured inside.
Only after emergency response crews with protective equipment have had a chance to test the air inside the facility and confirm it is safe to do so should employees return to their workstations.
Cyanide Gas
The following is an extremely difficult text to write. In case of a cyanide gas release, employees must be instructed to evacuate, and while they are evacuating, they should make an attempt to tell as many co-workers as possible. They must not stay around to warn or rescue others unless, of course, they have a gas-tight chemical protection suit on and self-contained breathing apparatus. Some facilities may want to have such suits and SCBA stored outside the facility to allow for rapid deployment after an evacuation.
If a co-worker has collapsed and they suspect it was caused by cyanide gas exposure, they must NOT attempt to make a rescue. They need to leave the area immediately. This is advice that is based upon experience at a plating shop many years ago, where several workers were killed by cyanide gas exposure while attempting to rescue fallen friends.
Fires and Cyanide Gas
Cyanide gas is flammable and will add to the heat load of any fire involving this gas. Fires where cyanide compounds are present should not be extinguished using water or carbon dioxide, as either of these reacts with cyanide to produce flammable and toxic gas. The best extinguishing media are powder or foam.
Closed containers of cyanide may be cooled with water (foam has little or no cooling effect) as long as there is a low possibility of water and cyanide mixing. If there is no risk to the surroundings, firefighters may need to allow the fire to burn itself out from a safe distance.
Exposure (But Not Over-Exposure) Via Eye Contact
For eye contact, contact lenses need to be immediately removed prior to the use of an eyewash station. Never reuse these, even if they are not disposable.
Throw them into the hazardous waste bag. Use the eyewash station for at least 15 minutes to wash out all traces of chemicals. Always go to an eye doctor as soon as you are done with the eyewash station.
Cyanide Over-Exposure
A key to emergency response for cyanide exposure is to realize that:
- Cyanide exposure is best treated with specific antidotes and supportive medical care in a hospital setting.
- Time is extremely critical; first aid and medical treatment must begin as soon as possible.
- The first aid to be conducted by a nonmedically trained person is only a stopgap measure while trained professionals are called in.
- You must be wearing the appropriate personal protective equipment while conducting the emergency response procedures detailed below.
Exposure Via Gas, Ingestion, or Skin Contact
The following are actions that can mitigate the exposure or protect you from exposure by the victim. Administer first aid while any of the below activities are conducted.
- If it is easy to do, remove a victim of cyanide gas exposure to fresh air, but do not allow this to delay the application of first aid.
- Do not attempt mouth-to-mouth resuscitation, as this may expose you to cyanide.
- Examine the victim for contaminated clothing, which will need to be removed. Contaminated clothing needs to be placed inside a poly bag, sealed, and then re-bagged and disposed of as hazardous waste.
- Do not pull contaminated clothing over the head of the victim, as this can cause additional exposure!
- Remove any jewelry that has been contaminated.
- Any skin areas that have been in contact with cyanide will need to be washed with soap and water.
First Aid
The following are first-aid steps to consider:
- Make sure a doctor or emergency response team has been called and that they have been informed that they are responding to a cyanide exposure.
- Have a co-worker administer artificial respiration (not mouth-to-mouth) while you administer amyl nitrite pearls from the Cyanide Antidote Kit.
- Evaluate if the victim has had any skin exposure and respond accordingly.
Amyl Nitrite
The Cyanide Antidote Kit contains numerous items that are to be used by a trained medical professional. Only the amyl nitrite is for use by an untrained responder. The
package contains 12 pearls, and each is to be used for a total of three minutes, giving you a total of 36 minutes of treatment. That means that the emergency response team needs to know that they need to arrive within 30 minutes of an emergency call.
The Kit contains information on the use of the amyl nitrite in two places (under the lid and in the package in loose leaf format.
The amyl nitrite pearls are contained in a thin glass tube covered in paper (photo). Treatment consists of:
- Crushing the pearl in a handkerchief. In the absence of a cloth or handkerchief, use your bare fingers, but make sure you don’t breathe the fumes yourself (they can make you dizzy).
- Holding the broken ampoule under the victim’s nose for 15 seconds.
- Holding the amyl nitrite away from the nose for 15 seconds. This is extremely important because continuous exposure to amyl nitrite may kill the victim!
- Repeating for a total of three minutes before changing to a new broken pearl and repeating the treatment.
If the victim is unconscious and not breathing, the pearl needs to be placed inside an oxygen mask while artificial respiration is conducted. If no oxygen mask is available, place a handkerchief with the amyl nitrite over the nose and apply artificial respiration (not mouth-to-mouth).
It is a darn good idea to practice first aid treatment using outdated or “pretend” amyl nitrite pearls.
The contents of the Cyanide Antidote Kit are subject to expiration (the date is on the label on the front of the Kit). Make sure it is replaced before the expiration date. The maximum storage temperature is 25°C (77°F), so it needs to be stored in an air-conditioned environment.
Frank Altmayer is a Master Surface Finisher and an AESF Fellow who is the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in metal finishing. He was the recipient of the AESF Past Presidents Award, NAMF Award of Special Recognition, AESF Leadership Award, AESF Fellowship Award, Chicago Branch AESF Geldzahler Service Award, and NASF Award of Special Recognition.



































