The prices for metal anodes and metal salts have increased substantially, with little stability over the years.
 Stephen Rudy CEFIt is an unfortunate economic circumstance that mining sources, refiners, sellers, and end users must deal with. Electroplating baths each provide specific benefits that, regardless of cost, have become necessary for various on-demand finishes. These include decorative, functional, wear resistance, and corrosion protection. Nickel encompasses many plating systems, which are very important to many aspects of general metal finishing. Even though the prices of nickel anodes and related salts have increased sharply, the importance of nickel in plated finishes is still of great significance and demand. Let us review some pertinent facts regarding practical nickel electroplating. The information should balance or support the need for nickel electroplating, even if the finishing cost is unstable. (Image courtesy of broadwaybrass.co.uk)
Stephen Rudy CEFIt is an unfortunate economic circumstance that mining sources, refiners, sellers, and end users must deal with. Electroplating baths each provide specific benefits that, regardless of cost, have become necessary for various on-demand finishes. These include decorative, functional, wear resistance, and corrosion protection. Nickel encompasses many plating systems, which are very important to many aspects of general metal finishing. Even though the prices of nickel anodes and related salts have increased sharply, the importance of nickel in plated finishes is still of great significance and demand. Let us review some pertinent facts regarding practical nickel electroplating. The information should balance or support the need for nickel electroplating, even if the finishing cost is unstable. (Image courtesy of broadwaybrass.co.uk)
Combining the Inherent Properties of Nickel
For industrial purposes, almost any metal can be nickel electroplated. The inherent properties of nickel can be combined with the unique properties of other metals. Some examples of metals are commonly plated with nickel steel (high strength), brass (easily bent and formed), aluminum (impact extrusion), and die castings (design flexibility). Plastics, which have been treated for conductivity, are also commonly nickel-plated. Nickel can be directly plated over several metals. This is typically performed directly over steel, brass, and copper. In some cycles, appropriate immersion treatments or pre-plate deposits precede nickel. This is especially prevalent when plating a copper strike and copper plate over zinc parts before nickel. Because of its unique electropositive nature, aluminum must first be conditioned by immersion zincating before plating either electrolytic or electroless nickel.
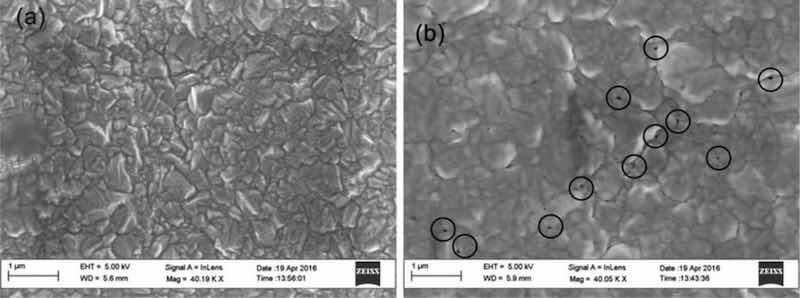 SEM images of nickel electrodeposited from a Watts Bath.
SEM images of nickel electrodeposited from a Watts Bath.
Applying a suitable nickel deposit can significantly benefit the part’s ultimate quality of the finish and meet or exceed specifications. These advantages reduce related manufacturing costs, improve marketability, and increase production throughput. Depending on the finishing requirements or service life of parts, nickel contributes the following advantages:
Good electrical conductivity, low coefficient of thermal expansion, magnetic, and good heat conduction. Nickel can be electroplated as a soft deposit (not far from copper) or almost as hard as chrome. Nickel can be electroplated to almost any desired deposit hardness between this wide range. Aesthetically, nickel can be plated in decorative applications to achieve a wide range of brightness and leveling while retaining sufficient deposit ductility.
Finished parts can be assembled or mechanically formed into selected commercial products. Along with these benefits, duplex nickel forms an excellent corrosion barrier, especially in the plating of exterior automotive parts. Nickel forms an important barrier to prevent the migration of either copper or zinc on tin-plated parts. In engineered finishes, nickel decreases contact, resistance, and friction, improves solder ability and brazing, and improves resistance to galling and wear. Plating with nickel can salvage worn or miss-machined parts. Almost all nickel deposits can be machined. Incorporating nickel into the deposit in place of solid metal reduces some manufacturing costs.
Rapid Leveling, Filling Voids, Eliminating Peaks and Valleys
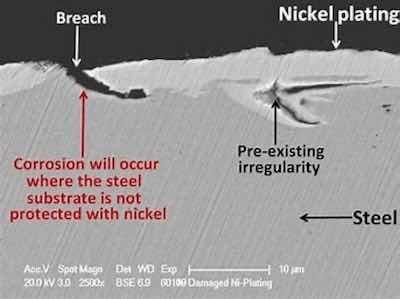 The coating protection a nickel electrodeposit for steel.As mentioned, nickel can be plated to provide rapid leveling, filling voids, and eliminating microscopic “peaks and valleys” while plating a relatively thin deposit. This is due to high-purity organic additives sometimes referred to in general terms as “acetylenics”. For this reason, the base metal, especially rough, may not have to be mechanically polished, buffed, or mass finished. If this is acceptable, cost savings may be realized in preparing the surface for plating. Nickel can be electroplated from various specific bath formulations (usually Watts and sulfamate types) to develop deposits that range from flat to dull to semi-bright to bright. Sulfamate nickel provides a low-stress deposit with very good mechanical properties. The high current efficiency permits rapid deposition of low-stress nickel. This allows the finisher to provide nickel deposits that meet engineering requirements, corrosion protection, and aesthetic preferences.
The coating protection a nickel electrodeposit for steel.As mentioned, nickel can be plated to provide rapid leveling, filling voids, and eliminating microscopic “peaks and valleys” while plating a relatively thin deposit. This is due to high-purity organic additives sometimes referred to in general terms as “acetylenics”. For this reason, the base metal, especially rough, may not have to be mechanically polished, buffed, or mass finished. If this is acceptable, cost savings may be realized in preparing the surface for plating. Nickel can be electroplated from various specific bath formulations (usually Watts and sulfamate types) to develop deposits that range from flat to dull to semi-bright to bright. Sulfamate nickel provides a low-stress deposit with very good mechanical properties. The high current efficiency permits rapid deposition of low-stress nickel. This allows the finisher to provide nickel deposits that meet engineering requirements, corrosion protection, and aesthetic preferences.
The Woods Strike effectively activates stainless steel for subsequent nickel plating. Credit goes to the late Technical Director at Hill Cross Company, Donald Woods, who developed this important activation process. As mentioned earlier, Duplex nickel promotes exceptional corrosion protection by plating a balanced ratio of a special semi-bright and bright nickel. The semi-bright nickel deposit structure is columnar; the bright nickel deposit structure is laminar. This unique balance of deposit structures enables the semi-bright nickel deposit to be cathodic to the bright nickel deposit, acting as a corrosion inhibitor should the bright nickel deposit become fractured. The Step Test is a specific quality control procedure for this application. Watts baths containing modified organic additives have successfully replaced cyanide copper strikes over zincated aluminum in recent years.
Notably, nickel deposits are flexible to the coating appearance: dull, semi-bright, or bright.
Decorative trivalent or hexavalent chrome continues to be a specified finish over nickel. The chrome topcoat enhances the overall appearance and maintains an excellent scratch-resistant, hard finish. Although we commonly refer to the bright chrome finish, it is primarily nickel (usually bright) with a thin chrome flash. Combining these deposits gives the assembled parts a pleasing appearance, exceptional corrosion, and wear resistance. The combination of bright nickel and flash chrome has been the best selection for plating parts subject to outdoor exposure and automotive interior and exterior finishes for several decades.
Stamped, Drawn, or Formed in a Variety of Shapes
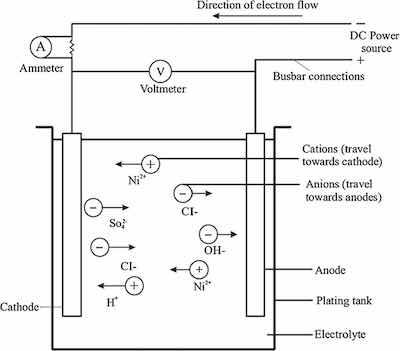 Diagram of a nickel electroplating plating system illustrating operating dynamics.Nickel can be electroplated as a very ductile deposit. Combined with good base metal conditioning and any preplate deposits, the finished items can be stamped, drawn, or formed in various shapes. This is very common in the strip plating of continuous coils that will be used to manufacture different consumer and industrial goods. In this application, the organic brightener and leveling additives may be kept at lower levels to achieve the required ductility. The final aesthetic appearance of nickel occurs in a short buffing cycle before optional chrome plating. Parts that require exceptional brightness and leveling may be stamped before the plating cycle. Heat-treating dull nickel increases deposit ductility by up to 50%. However, this is at the expense of significant hardness and tensile strength decreases.
Diagram of a nickel electroplating plating system illustrating operating dynamics.Nickel can be electroplated as a very ductile deposit. Combined with good base metal conditioning and any preplate deposits, the finished items can be stamped, drawn, or formed in various shapes. This is very common in the strip plating of continuous coils that will be used to manufacture different consumer and industrial goods. In this application, the organic brightener and leveling additives may be kept at lower levels to achieve the required ductility. The final aesthetic appearance of nickel occurs in a short buffing cycle before optional chrome plating. Parts that require exceptional brightness and leveling may be stamped before the plating cycle. Heat-treating dull nickel increases deposit ductility by up to 50%. However, this is at the expense of significant hardness and tensile strength decreases.
Nickel can be electroplated to meet any thickness requirement. Industrial-based coatings usually require 0.005 – 0.020 inch. For decorative purposes, nickel thickness may range from 0.0003 – 0.001 inch (or up to one mil). As indicated by the application ranges, a minimum should be plated to meet the intended use of finished parts. As a guide, 1 lb of plated nickel is required for every 22 ft2 of intended parts coverage. Depending on the nickel bath and plating parameters, the deposit tensile strength can range from 50 to 220 thousand lb / inch2.
Nickel anodes are expensive. Be certain a certificate of analysis confirms the quality. Anything less than sufficient purity material could result in severe contamination of the nickel bath. A typical assay is: Nickel (99.950%), Cobalt (0.03%), Copper (0.005%), Carbon (0.001%), Iron (0.001%), Sulfur (0.01). Anodes are provided in various shapes, including spears, buttons, rounds (sulfur-containing S and sulfur-free R), and chunks.
There are many applications for electroplating nickel, using several process baths. The demand for nickel electroplating continues to be relatively strong. Although the prices for nickel anodes and salts have markedly risen, the consumer market for plated finishes keeps this plating service very active. Original and after-market automotive finishes have “re-discovered” bright nickel/chrome finishes. The decorative plumbing industry is very positive on nickel/chrome and brushed nickel finishes. Clothing and apparel manufacturers now feature nickel finishes (ex., oxidized, brushed, under flash brass, or gold). Nickel anodes and salts may not be cheap (compared to years past), but decorative and industrial finishes for nickel are still in strong demand.
Stephen F. Rudy, CEF, is president of Chem Analytic and has written extensively about the finishing industry. Visit www.chemanalytic.com or call him at 917-604-5001.



































